Pharmacotherapy for Chronic Obstructive Pulmonary Disease
Andrew Schaefer, Antwon Taylor, Zhiqian Wu and Desuo Wang*
School of Pharmacy, Philadelphia College of Osteopathic Medicine, US
Submission: June 16, 2022; Published: June 16, 2022;
*Corresponding author: Desuo Wang, School of Pharmacy, Philadelphia College of Osteopathic Medicine, US
How to cite this article:Andrew S, Antwon T, Zhiqian W, Desuo W. Pharmacotherapy for Chronic Obstructive Pulmonary Disease. Glob J Pharmaceu Sci. 2022; 10(1): 555776. DOI: 10.19080/GJPPS.2022.10.555776.
Keywords: Pharmacotherapy; Pulmonary disease; Public health; Healthcare; Pharmacological treatments
Abbreviations: COPD: Chronic Obstructive Pulmonary Disease; SABAs: Short Acting Beta2-Agonists
Introduction
Chronic obstructive pulmonary disease (COPD) increases public health concern, and its management is a challenge for healthcare professionals. According to the American Lung Association, COPD is the fourth leading disease-caused death in the United States, and treatment options are limited to alleviation of symptoms, mitigation of disease progression and exacerbation, and improvement on patient quality of life. GOLD [1-4] it was reported that the direct and indirect costs of COPD total over $50 billion in the United States alone Guarascio [5] and the 20-year (2019-2039) discounted direct medical costs attributable to COPD are estimated to be more than $800 billion Zafari [6]. This area needs further research as the projections for cases of COPD are increasing globally, due to aging populations as well as continued introduction of risk factors that precipitate the disease CDC [7]. COPD is characterized by tenacious dyspnea, cough, sputum production, and airflow limitation that is due to airway impediments usually caused by significant exposure to noxious particles or gases. Barnes [8] this chronic airway obstruction is instigated by bronchiolitis and is perpetuated with emphysema progression, but the extent of each varies between patients, making targeted therapy and diagnosis a difficult task. Smoking is the main risk factor. for COPD, so smoking cessation is crucial in order to maximize the outcomes of therapy. GOLD [1] once a diagnosis of COPD is made and the class of severity is determined, pharmacological treatment can be initiated in an effort to: reduce the symptoms, reduce the frequency and severity of exacerbations, and improve exercise tolerance and overall health status. GOLD [1] the therapeutic regimens are individualized and escalated based on the symptoms, diagnosis and the severity of the disease as well as the presence of comorbidities. The major pharmacologic treatment classes are selective beta-2 agonists, antimuscarinic agents, methylxanthines, and anti-inflammatory agents such as inhaled corticosteroids and selective PDE4 inhibitors. The oral corticosteroids, antibiotics and mucolytics/antioxidants are added for management of the exacerbations. [1,3,8] Patient management of pharmacological treatment is the focus of this review, which will break down and evaluate the efficacy of the current pharmacotherapy options across groups.
Pharmacological Agents Used for Treatment of COPD
Bronchodilators are comprised of different duration beta2-agonists and antimuscarinics. Short acting beta2-agonists (SABAs), such as albuterol, are used on a regular and as-needed basis for acutly improving ventilation (measured as FEV1) and relieving the shortness of breath. Sestini [9] The duration of SABAs are typically 4-6 hours, thus requiring continued usage throughout the day. Long acting beta2-agonists (LABAs), such as salmeterol, have a duration of 12 or more hours and can be used alongside SABAs. LABAs provide twice daily dosing that significantly improves FEV1, increases lung volumes, reduces dyspnea and exacerbation rate as well as reduces the number of hospitalizations and the costs of managements. [10,11] Indacaterol, oladaterol and vilanterol are LABAs which have a duration of 24 or more hours, but the use of LABAs as monotherapy is not recommended. Bell [12] treatment with beta-2 agonists can produce adverse effects such as cardiac rhythm disturbances and tremor especially with higher doses. Prior asthma research has raised concerns about possible loss of lung function and increased mortality with the use of beta bronchodilators, but this has not been reported with COPD.
[10,13,14] recently preclinical studies indicated that long term use of β-2-adrenoceptor agonists may help to stimulate glucose uptake in skeletal muscle and improve glucose homeostasis, which is beneficial for insulin resistance diabetic animal models Kalinovich [15].
Similarly, antimuscarinic therapy are comprised of short acting muscarinic antagonists (SAMAs, such as ipratropium) and long-acting muscarinic antagonists (LAMAs, such as tiotropium), which block acetylcholine-mediated broncho constricting effects caused by activating muscarinic receptors in airway smooth muscle. Long-acting muscarinic antagonists (LAMAs), such as tiotropium, have a prolonged bronchodilator effect due to their binding at M3 muscarinic receptors. Melani [16] when comparing the therapeutic efficacy, more favorable improvements in lung function with antimuscarinics rather than beta2 agonists. Appleton [1,18,19] treatment with antimuscarinics are typically associated with anticholinergic side effects, such as constipation and drying of mucus membranes. Gelb [20] in patients with advanced COPD, these two classes of bronchodilator medications are frequently used in combination. [1,3,21]. Methylxanthines are utilized when patients fail to respond to treatment options targeting adrenergic and/or muscarinic pathways. The addition of theophylline to the LABA salmeterol has shown an improvement in FEV1 and breathlessness when compared to salmeterol alone. Ram [22] side effects such as nausea, vomiting, and headaches can occur within the therapeutic range of theophylline and the metabolism by cytochrome P450 introduces the possibility of significant medication interactions. The narrow therapeutic window of methylxanthines can lead to toxicities and ultimately prevents the widespread usage in treatment of COPD. More recent trial results failed to confirm the therapeutic efficacy of theophylline Devereux [23]. Selective phosphodiesterase-4 (PDE4) inhibitors are used to reduce inflammation in patients who have a moderate to severe diagnosis and a history of exacerbations. The most commonly used PDE4 inhibitor roflumilast works by inhibiting the breakdown of intracellular cyclic AMP. [24,25] this oral medication can be taken once daily to improve lung function, in combination with systemic corticosteroids, LABAs, or fixed-dose LABA/ICS combinations. Calverley, Fabbri & Martinez [26,27,28] roflumilast causes more adverse effects than inhaled medications, but they tend to occur at the initiation of treatment and subside with time GOLD [1] The most common adverse effects are weight loss, nausea and dose-limiting emesis. The current recommendation is to use PDE4 inhibitors as an add-on medication. It is possible that early administration of roflumilast may slow the progression of the disease and reduce or avoid the chronic usage of oral corticosteroids in patients with severe COPD. Inhaled corticosteroids (ICS) are the most powerful anti-inflammatory agents, and they are always used in combination with beta2- agonists or antimuscarinic agents in the management of COPD. Their anti-inflammatory effects alone do not modify the longterm decline of FEV1 or mortality in patients with COPD, but they do provide benefit for patients with frequent exacerbations and exacerbation-related hospitalization. GOLD & Yang [1,29] ICS and LABA used in combination have shown to be more effective than either treatment alone in improving lung function, health status and reducing exacerbations. Nannini [30,31] risks associated with ICS use include increased prevalence of pneumonia and oral candidiasis among several other complications due to the immune suppression. GOLD & Yang [1,29] the discontinuation of ICS use is associated with withdrawal of lung function, along with increased symptoms and exacerbations Magnussen [32], which makes the decision of discontinuation to use difficult.
The purpose of this review is to highlight the clinical efficacy and place in therapy for the management of treatment for patients with COPD. In order to evaluate the current treatment options available for COPD their pulmonary efficacies are compiled in Table 1. Spirometry is still widely used in the diagnosis of COPD and FEV1 remains a constant in the evaluation of medication efficacy. The focus points for this review were on the current treatment options, and their respective clinically significant improvements in FEV1 among patients diagnosed with COPD. Data was collected using PubMed and the U.S. National Library of Medicine for clinical trials, and the corresponding trails are listed in Table 2.
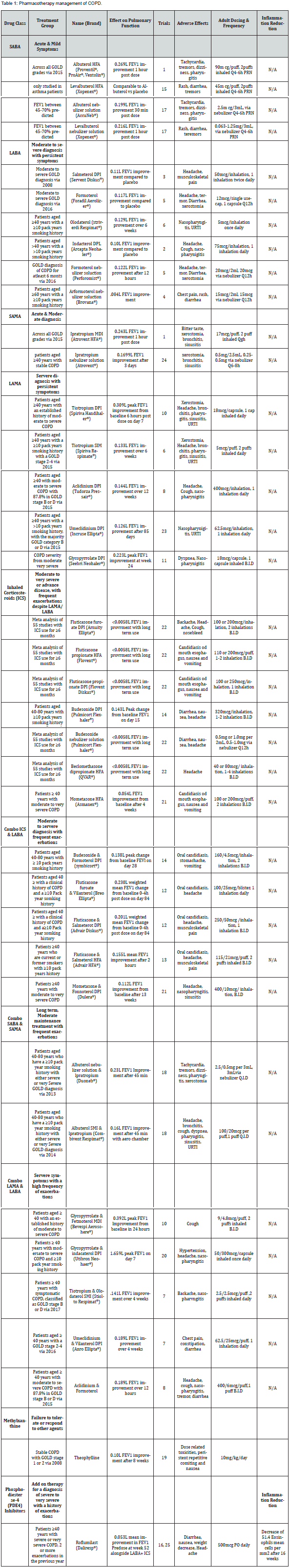
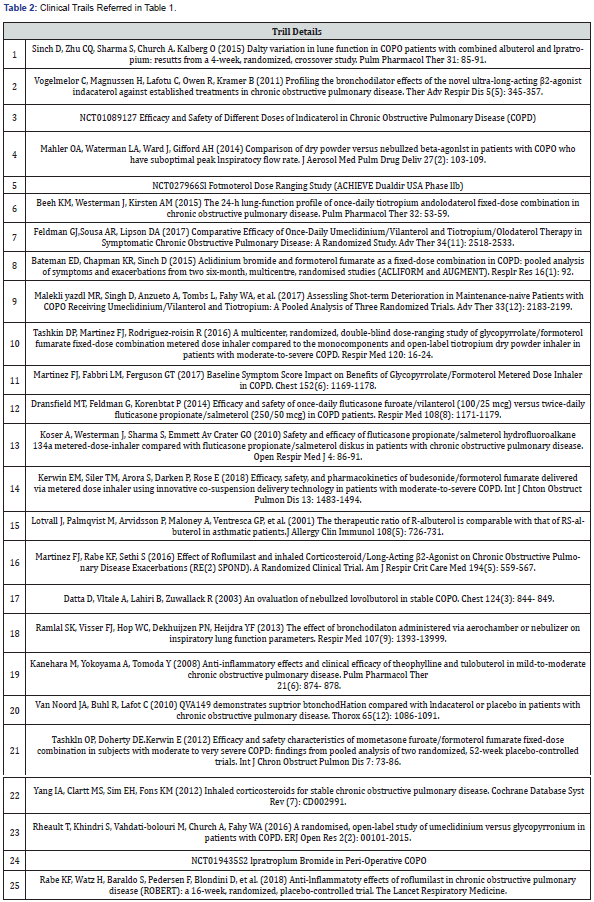
Since the incidence of COPD increases with age, smoking status, and the presence of comorbidities, these factors can certainly contribute to the findings of each study. As the GOLD guidelines have adapted to new research and improved over the years, the staging and recommended treatment options have coincided. This table references trials conducted over the past decade, and their treatment groups reflect the patient management options available at those times. Comorbidities, airflow limitation, history of exacerbations and other independent factors should always be considered in the current diagnosis and treatment of patients with COPD. This table has an emphasis upon FEV1 improvement as well as inflammation reduction for the purpose of determining the optimal management of treatment. It will serve as a reference in the analysis of recommended pharmacotherapy per the GOLD 2018 guidelines. GOLD [1] drug classes are listed beside their current place in therapy and individual therapies are separated by the specific treatment group evaluated in each respective study. Alongside the effect on pulmonary function, the corresponding trail data is numbered and listed at the end of this paper in Table 2. Common adverse effects as well as the dosage used to elicit the responses follow. Group A is excluded from the analysis due to the wide range of symptoms as well as treatment options
Pharmacotherapy management for GOLD group B
Initial treatment of patients diagnosed to Group B consists of long-acting bronchodilators (LABA or LAMA). Cochrane systematic reviews have established long-acting bronchodilators to be superior to their short acting counterparts. Appleton & Barr [1,3] if patients in this group suffer from persistent symptoms or exacerbations, only then they are recommended a combination LABA and LAMA. In reference to Table 1, olodaterol has shown the greatest improvement in FEV1 amongst LABAs and tiotropium amongst LAMAs. Olodaterol is highly selective with nearly full intrinsic activity at the Beta2 receptors and has been shown to effectively improve lung function and patient reported symptoms over 24 hours Bouyssou & Koch [34,35]. In the Beeh study Beeh [36] of olodaterol, the primary end point was FEV1 area under the curve response 0 to 24 hours after 6 weeks of treatment, resulting in an improvement of 0.129L from a once daily 5mcg dose. In the same study, 5mcg of tiotropium daily produced an improvement of 0.133L after 6 weeks which would lead to the assumption that they have similar effectiveness despite their different mechanism of action.
Tiotropium is traditionally dosed at 18mcg daily, and in the
Tashkin study Tashkin [37] it was administered open label as its
brand name Spiriva HandiHaler ® at that dosage. As a secondary
endpoint in this study, tiotropium resulted in a peak change in
FEV1 over 6 hours post-dose on day 7 of 0.309L. Although this
improvement appears proportional to the increase in dosage from
the Beeh [36] study, peak FEV1 over 3 hours a week 6 from 5mcg
olodaterol and 5mcg tiotropium resulted in 0.291L and .300L
respectively. These results lead to the conclusion that tiotropium
is slightly more efficacious than olodaterol in FEV1 improvement.
Based on this data, it would be reasonable to suggest that Group
B COPD patients start therapy with a LAMA such as tiotropium or
umeclidinium rather than a LABA. In a recent head-to-head study,
the efficacy of umeclidinium 62.5mcg was shown to be greater
than 18mcg tiotropium in terms of the least squares mean change
from baseline in trough FEV1. Feldman [38] In a following-up
study by Feldman [39], the efficacy of tiotropium and olodaterol
once-daily fixed-dose combination was analyzed versus the oncedaily
umeclidinium and vilanterol combination. This was the first
study to analyze the components when delivered through a single
device. Tiotropium/olodaterol was administered once daily via
the Respimat ®< inhaler, as two puffs of 2.5/2.5mcg. Umeclidinium
and vilanterol were administered once daily via the Ellipta
Pharmacotherapy management for GOLD group C
Patients diagnosed to Group C are initially started on a LAMA and if they suffer from increased exacerbations, therapy is extended to a LAMA plus LABA, or alternatively a LABA plus ICS. The preferred treatment in this group is LAMA and LABA, because ICS usage increases the risk of developing pneumonia in some cases. GOLD [1]. These treatment options do not differ much from the Group B therapy, besides the addition of ICS. When comparing LAMA and LABA against LABA and ICS treatments, Table 1 again demonstrates a greater improvement in FEV1 with glycopyrrolate and formoterol (LABA and LAMA) therapy rather than fluticasone furoate and vilanterol (ICS and LABA). The difference is greater than 100mL, but treatment with ICS may be more beneficial in certain patients with frequent exacerbations when considering time to onset. A 2017 Cochrane review of LAMA and LABA versus LABA and ICS analyzed eleven studies, which found fewer exacerbations and a larger improvement in FEV1 in the LAMA + LABA group, along with a lower risk of pneumonia. Horita [40]. These data support the current intensification of treatment for Group C with anti-inflammatory agents and indicate the importance of reducing chronic inflammation in the management of COPD. This may prompt the beneficial effects of using selective PDE4 inhibitor roflumilast in early stage of COPD patients. When comparing the optimum FEV1 values from Table 1, treatment with the LAMA tiotripium 18mcg once daily has a 0.309L improvement opposed to a 0.230L improvement with the combination LABA/ICS vilanterol and fluticasone furoate 25/100mcg once daily. Tashkin & Dransfield [37,41] these values point towards a better response with the monotherapy of a LAMA over the combination of LABA and ICS, but the frequency of exacerbations and individual patient quality of life must be taken into consideration when balancing the immediate improvement in ventilation and slowing down the inflammation-related disease progression and exacerbations. In a Cochrane database review of fluticasone furoate and vilanterol versus tiotropium, no statistically significant differences were found for the improvement of symptoms in COPD assessment Test (CAT) score nor FEV1 after 84 days. The authors Sliwka et al. [42] suggested that further trials with longer duration are needed to determine if there is an advantage of either therapy since the current data is not strong enough to establish differences in efficacy or equivalency. The introduction of a PDE4 inhibitor in the addition of a LAMA or LABA here may be an alternative route to explore for the treatment of further exacerbations in Group C rather than an ICS. Table 1 demonstrates the use of ICS results in minimal FEV1 improvement, specifically <0.0058L with long term use. Yang [29] roflumilast dosed at 500mcg once daily by mouth resulted in a FEV1 improvement of 0.053L, and significantly reduced the rate of moderate or severe exacerbations in patients with a history of more than three in the past year. Martinez [43] Use of roflumilast could potentially decrease the rate of progression from patients in Group C to Group D.
Pharmacotherapy management for GOLD group D
Current recommendations for patients in the Group D stage are dependent on the patient’s previous treatment. LABA and LAMA combination is the recommended starting therapy which is consistent with the previous treatment of choice in groups B and C. Group D patients have been shown to be at a higher risk of developing pneumonia when treated with ICS Crim et al. [44] due to the disease progression and the suppression of immune defense system of the body by chronic and large amount of steroid exposure. The risk of pneumonia is an important concern in the selection of treatment, but some patients in Group benefit from LABA and ICS use especially if they have been suspected of or have a history of asthma-COPD overlap. GOLD [1] after determining the proper initial treatment, any further exacerbations or persistent symptoms would escalate treatment to triple therapy of LAMA, LABA, and ICS. Only after triple therapy is roflumilast considered if the patient is still experiencing exacerbations. Specifically, in patients with an FEV1 < 50% predicted, chronic bronchitis, or particularly if they have had at least one hospitalization for an exacerbation in the previous year. Martinez, Martinez & Rabe [28,43,45] results of roflumilast (thirty-four randomized controlled trials) in patients including Group B-D stages showed a significant improvement in FEV1 by 0.0515L compared to placebo Chong [46], as shown in Table 1.
Anti-Inflammatory Therapy and COPD Management
The PDE4 inhibitor roflumilast is currently not recommended by the GOLD guidelines unless the patient reaches a Group D diagnosis. Previous studies have analyzed the effect of roflumilast in conjunction with traditional therapies, but its use alone for the prevention of COPD progression has not been evaluated. In the RE (2) SPOND trail, the difference in mean change from baseline in pre-dose FEV1 was 0.053L when orally taking roflumilast 500mcg once daily. Although this Martinez et al. [43] change in FEV1 is below the normally considered minimum clinically important difference of 100mL, it still provides considerable evidence for its use outside of a last line add on therapy. Donohue [47] in comparison to the FEV1 mean improvement of 0.047L by steroid fluticasone, this anti-inflammatory agent may provide more benefits than currently ascribed. Calverley et al. [48] several studies have focused on the use of roflumilast in the prevention of exacerbations and have exhibited the efficacy which supports its recommended usage. In a 2017 Cochrane Library review of PDE4 use in COPD, the number needed to treat for a single person to be exacerbation-free was found to be 20 (95% CI 16 to 26) Chong [46] these discoveries suggest that PDE4 inhibitors in patients with COPD are acting independently of the other treatments and could be supportive of its broad anti-inflammatory efficacy Fabbri et al. [27]. The recently published ROBERT trial Rabe et al. [49] looked specifically at the anti-inflammatory effects of roflumilast in COPD. Bronchial biopsies were taken at baseline and week 16 to evaluate: CD8, CD68, CD4, CD45, neutrophils, eosinophils per mm2 in the submucosa, as well as CD8 and CD68 cells in the bronchial epithelium. The primary endpoint of the study was the mean change from baseline in CD8 cells, but the results against placebo were not statistically significant. As a secondary outcome, the decrease in eosinophils was significantly greater than placebo as shown in Table 1 by a value of 51.4 mean cells per mm2. This decrease of eosinophil Rabe et al. [49] counts in not only the bronchial biopsy specimens but also the induced sputum samples presents a new opportunity to evaluate the efficacy of different COPD treatments. The findings of ROBERT trial that blood eosinophilia counts do not necessarily reflect the lung eosinophilia as reported by Kolsum et al. [50]. Specific studies to determine eosinophil count of both blood and lung biopsies in COPD patients could help in the diagnosis as well as pharmacotherapy.
Summary
The treatment of COPD has progressed with the increased data collected on bronchodilators and anti-inflammatory therapies. GOLD guidelines have improved upon their diagnosis and treatment algorithms to better manage this chronic illness. With the current available treatment, COPD is still not a reversible or curable disease. New treatment options and studies must continue to understand the pathophysiology in order to advance patient care outcomes. Current therapy is able to help alleviate and manage symptoms. The use of PDE4 inhibitors may be underutilized in the treatment of patients in Groups B & C. Future studies should consider a more extensive approach over the disease progression and focus on the long-term effects of treatment options as well as developing new treatment for inflammatory markers. In addition, more specific studies based upon gender, age, and race are needed to help better identify which patients benefit most from the treatment algorithms.
References
- (2018) GOLD: Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD).
- Mirza S, Clay RD, Koslow MA, Scanlon PD (2018) COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin Proc 93(10): 1488-1502.
- Gupta N, Malhotra N, Ish P (2021) GOLD 2021 guidelines for COPD - what's new and why. Adv Respir Med 89(3): 344-346.
- https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm.
- Guarascio AJ, Ray SM, Finch CK, Self TH (2013) The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinico Economics and outcomes research CEOR 5: 235-245.
- Zafari Z, Li S, Eakin MN, Bellanger M, Reed RM (2021) Projecting Long-term Health and Economic Burden of COPD in the United States. Chest 159(4): 1400-1410.
- https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_06_tables.pdf.
- Barnes PJ (2017) in Hilal-Dandan R, Knollman B, Brunton L Goodman and Gilman's The Pharmacological Basis of Therapeutics, (13th edn), Pulmonary Pharmacology, McGraw-Hill Education, New York, United States.
- Sestini P, Renzoni E, Robinson S, Poole P, Ram FS (2002) Short-acting beta 2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev (4): CD001495.
- Kew KM, Mavergames C, Walters JA (2013) Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 10(10): CD010177.
- Bourbeau J, Bafadhel M, Barnes NC (2021) Benefit/Risk Profile of Single-Inhaler Triple Therapy in COPD. Int J Chron Obstruct Pulmon Dis 16: 499-517.
- Bell AD, McIvor RA (2007) The SMART study. Can Fam Physician 53(4): 687-688.
- Dahl R, Chung KF, Buhl R (2010) Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 65(6): 473-479.
- McGarvey L, Niewoehner D, Magder S (2015) One-Year Safety of Olodaterol Once Daily via Respimat(R) in Patients with GOLD 2-4 Chronic Obstructive Pulmonary Disease: Results of a Pre-Specified Pooled Analysis. COPD 12(5): 484-493.
- Kalinovich A, Dehvari N, Åslund A (2020) Treatment with a β-2-adrenoceptor agonist stimulates glucose uptake in skeletal muscle and improves glucose homeostasis, insulin resistance and hepatic steatosis in mice with diet-induced obesity. Diabetologia 63(8): 1603-1615.
- Melani AS (2015) Long-acting muscarinic antagonists. Expert Rev Clin Pharmacol 8(4): 479-501.
- Appleton S, Jones T, Poole P (2006) Ipratropium bromide versus long-acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 19(3): Cd006101.
- Vogelmeier C, Hederer B, Glaab T (2011) Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 364(12): 1093-1103.
- Decramer ML, Chapman KR, Dahl R (2013) Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med 1(7): 524-533.
- Gelb AF, Tashkin DP, Make BJ (2013) Long-term safety and efficacy of twice daily aclidinium bromide in patients with COPD. Respir Med 107(12): 1957-1965.
- Oba Y, Keeney E, Ghatehorde N, Dias S (2018) Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis. Cochrane Database Syst Rev 12(12): CD012620.
- Ram FS, Jones PW, Castro AA (2002) Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002(4): CD003902.
- Devereux G, Cotton S, Fielding S (2018) Effect of Theophylline as Adjunct to Inhaled Corticosteroids on Exacerbations in Patients With COPD: A Randomized Clinical Trial. JAMA 320(15):1548-1559.
- Wang D, Cui X (2006) Evaluation of PDE4 inhibition for COPD. Int J Chron Obstruct Pulmon Dis 1(4): 373-379.
- Rabe KF (2011) Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol 163(1): 53-67.
- Calverley PM, Rabe KF, Goehring UM, (2009) Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 374(9691): 685-694.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL (2009) Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 374(9691): 695-703.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, et al. (2015) Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 385(9971): 857-866.
- Yang IA, Clarke MS, Sim EH, Fong KM (2012) Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Sys Rev 7(7): CD002991.
- Nannini LJ, Lasserson TJ, Poole P (2012) Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 9(9): CD006829.
- Nannini LJ, Poole P, Milan SJ, Kesterton A (2013) Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 8(8): CD006826.
- Magnussen H, Disse B, Rodriguez-Roisin R (2014) Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 371(14): 1285-1294.
- Barr RG, Bourbeau J, Camargo CA, Ram FS (2005) Inhaled tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 18(2): CD002876.
- Bouyssou T, Casarosa P, Naline E (2010) Pharmacological characterization of olodaterol, a novel inhaled beta2-adrenoceptor agonist exerting a 24-hour-long duration of action in preclinical models. J Pharmacol Exp Ther 334(1): 53-62.
- Koch A, Pizzichini E, Hamilton A (2014) Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat® versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis 9: 697-714.
- Beeh KM, Westerman J, Kirsten AM (2015) The 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm Pharmacol Ther 32: 53-59.
- Tashkin DP, Martinez FJ, Rodriguez-roisin R (2016) A multicenter, randomized, double-blind dose-ranging study of glycopyrrolate/formoterol fumarate fixed-dose combination metered dose inhaler compared to the monocomponents and open-label tiotropium dry powder inhaler in patients with moderate-to-severe COPD. Respir Med 120: 16-24.
- Feldman G, Maltais F, Khindri S (2016) A randomized, blinded study to evaluate the efficacy and safety of umeclidinium 62.5μg compared with tiotropium 18μg in patients with COPD. Int J Chron Obstruct Pulmon Dis 11: 719-730.
- Feldman GJ, Sousa AR, Lipson DA (2017) Comparative Efficacy of Once-Daily Umeclidinium/Vilanterol and Tiotropium/Olodaterol Therapy in Symptomatic Chronic Obstructive Pulmonary Disease: A Randomized Study. Adv Ther 34(11): 2518-2533.
- Horita N, Goto A, Shibata Y (2017) Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2(2): CD012066.
- Dransfield MT, Feldman G, Korenblat P (2014) Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25mcg) versus twice-daily fluticasone propionate/salmeterol (250/50mcg) in COPD patients. Respir Med 108(8): 1171-1179.
- Sliwka A, Jankowski M, Gross-sondej I, Storman M, Nowobilski R, et al. (2018) Once-daily long-acting beta₂-agonists/inhaled corticosteroids combined inhalers versus inhaled long-acting muscarinic antagonists for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 8(8): CD012355.
- Martinez FJ, Rabe KF, Sethi S (2016) Effect of Roflumilast and Inhaled Corticosteroid/Long-Acting β2-Agonist on Chronic Obstructive Pulmonary Disease Exacerbations (RE(2) SPOND). A Randomized Clinical Trial. Am J Respir Crit Care Med 194(5): 559-567.
- Crim C, Dransfield MT, Bourbeau J (2015) Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc 12(1): 27-34.
- Rabe KF, Calverley PMA, Martinez FJ, Fabbri LM (2017) Effect of roflumilast in patients with severe COPD and a history of hospitalisation. Eur Respir J 50(1): 1700158.
- Chong J, Leung B, Poole P (2020) Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 5(5): CD002309.
- Donohue J (2005) Minimally clinically important differences in COPD lung function. COPD 2: 111-124.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356(8): 775-789.
- Rabe, KF, Watz H, Baraldo S, Pedersen F, Biondini D, et al. (2018) Anti-inflammatory effects of roflumilast in chronic obstructive pulmonary disease (ROBERT): a 16-week, randomized, placebo-controlled trial. The Lancet Respiratory Medicine.
- Kolsum U, Damera G, Pham TH (2017) Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J Allergy Clin Immunol 140(4): 1181-1184.






























