Development of Tablets Based on Lannea Microcarpa Engl. Et K. Krause (Anacardiaceae) Extracts for Arterial Hypertension Therapy
Salfo Ouédraogo1*, Josias BG Yaméogo2,3, Bavouma C. Sombié2, Hermine Zime Diawara2, Mathieu Nitiéma1, Lazare Belemnaba1, Tata Kadiatou Traoré2, Sylvin Ouédraogo1 and Rasmané Semdé1
1Département de médecine et pharmacopée traditionnelles-pharmacie (MEPHATRA-PH), Institut de recherche en sciences de la santé, Burkina Faso
2Laboratoire du Développement des médicaments (LADME), Ecole doctorale de la santé, Université Joseph Ki-Zerbo, Burkina Faso
3Laboratoire National de Santé Publique, Ministère de la santé, 09 BP 24 Ouagadougou 09, Burkina Faso
Submission: August 30, 2021; Published: October 05, 2021
*Corresponding author: Salfo Ouédraogo, Département de médecine et pharmacopée traditionnelles-pharmacie (MEPHATRA-PH), Institut de recherche en sciences de la santé, Burkina Faso
How to cite this article:Salfo Ouédraogo, Josias BG Yaméogo, Bavouma C. Sombié, Hermine Zime Diawara, Mathieu Nitiéma, et al. Development of Tablets Based on Lannea Microcarpa Engl. Et K. Krause (Anacardiaceae) Extracts for Arterial Hypertension Therapy. Glob J Pharmaceu Sci. 2021; 9(1): 555752. DOI: 10.19080/GJPPS.2021.09.555752.
Abstract
In context of hypertension, research in African traditional medicine allows the identification of safe and effective recipes that lead to the development of phytomedicines or the isolation of molecules that can be used in managing of hypertensive patients.
Objectives: This study aims at formulating and evaluating tablets based on Lannea microcarpa Engl. et K. Krause (Anacardiaceae) extracts for arterial hypertension therapy.
Methodology: Formulations of conventional release tablets containing 250mg and 500mg of extract were made. Several excipients were tested in several steps and wet granulation was performed. A formulation containing a diluent/disintegrant (corn starch), a binding agent (PVP K30) and a lubricant (magnesium stearate) was made. Then a flow agent (colloidal silica) was associated with the formulations of the first step. Finally, the colloidal silica of the previous formulations has been replaced by an anti-adhesive, the talc.
Discussion: The average masses of the 250 mg tablets varied from 263.187 mg ± 16.4 to 379.979 ± 13.1 with coefficients of variation between 2.72% and 6.23%. The average masses of the 500mg tablets varied from 561.047mg ± 15.13 to 783.388 ± 33.82 with coefficients of variation between 2.05% and 4.95%. These tablets had disintegration times of less than 15 minutes. The friability indices (< 1%) of the tablets of formulations FA6, 11, 17 and 18 as well as FB1, 2, 7, 8 and 15 alone complied with the specifications of the European Pharmacopoeia 10th edition. The mean contents were 0.378900 ± 0.010609 mg gallic acid equivalent (GAE)/tablet and 0.757 0.0211 mg GAE/tablet, respectively for the 250mg and 500mg tablets. The individual content of each tablet unit of both strengths was between 85 percent and 115 percent of the mean content and complied with the requirements of the European Pharmacopoeia 10th edition. These analyses led to the choice of FA6 and FB2 as the optimal formulation.
Conclusion: These results show the feasibility of the extracts of the plant in pharmaceutical form for the treatment of arterial hypertension.
Keywords: Arterial hypertension; Extracts; Formulations; Lannea macrocarpa; Tablets
Introduction
High blood pressure (HBP) is leading chronic disease in the world [1]. It is currently a public health problem worldwide, because of its burden. It also increases the risk of stroke, coronary heart disease, heart failure, kidney failure and cognitive impairment [2]. Its management considers the environmental factors, the genetic capital, the medical staff with all the available therapeutic arsenal (drug treatments, biological analyses,traditional and alternative medicine etc.) [3]. In terms of drug treatment, there are five main classes of antihypertensive drugs that have demonstrated their effectiveness in terms of morbidity and mortality in managing uncomplicated essential hypertension. These include thiazide diuretics, beta-blockers, angiotensin II antagonists (ARBs), ACE inhibitors and calcium channel blockers (CCBs). For these treatments, it is often necessary to take several combinations of drugs under specific conditions (Long-term treatment, restrictive schedules, etc.) [4]. Therefore, solid oral forms that are easy to use, and store improve compliance and offer significant advantages. Despite the recommendations of national and international learned societies on the management of hypertension, it remains insufficiently detected, treated, and controlled, indicating that the impact of these recommendations remains insufficient or even weak in the general population [5,6].
Despite considerable progress in managing hypertension in recent years, a large proportion of hypertensive subjects still have uncontrolled hypertension, especially in Africa. This is justified by problems of fragmentation, distribution, combined with difficulties in terms of availability, geographical accessibility and affordability resulting from an increased lack of universal health coverage. Because of these socio-cultural and economic barriers, Africa is becoming more active in developing traditional medicine in the fight against hypertension [7]. The research developed in this sense aims to enhance the value of traditional medicine through actions such as the involvement of herbal healers, the discovery of new molecules, the formulation of accessible and usable drugs to treat priority diseases [8]. These plants are used by traditional practitioners in old liquid forms derived from macerations, infusions, and decoctions etc., with stability problems. Possibilities of formulation in several forms (capsules, tablets, creams, gels, ointments etc.) are studied. Some are more innovative in modified release or microcapsule forms [9]. Among these conventional forms, solids such as capsules and tablets have higher stability, are easier to standardize [10] and represent two-thirds of the world market for drugs [11]. Lannea microcarpa Engl. et K. Krause (Anacardiaceae), a medicinal plant found in the Sahelo-sudanian and Sudanian savannahs, [12] is one of the antihypertensive medicinal plants used by herbal healers [13,14], and which present some efficacy and safety. Indeed, ethnobotanical studies have indicated the use of its trunk bark in the form of decoction in the treatment of hypertension in Burkina Faso [14-16]. Experimental studies (in vitro, ex vivo and in vivo) have demonstrated the safety and Anti-hypertensive properties of freeze-dried aqueous decoct of the bark of the plant trunk [17- 22]. Also studies of physicochemical characterization, quality control and galenic formulation have allowed the development of capsule forms based on standardized extracts dosed at 250mg [23-25]. Characterization studies showed that the freezedried aqueous extract was hygroscopic and had poor physicomechanical properties [23]. This justified the initial choice of using the capsule form in order to ensure protection against moisture. Moreover, the process of obtaining the capsules is simple and less expensive. However, the development of a capsule form dosed at 500 mg of extract, for use in adult subjects, could not be achieved because of technological difficulties related to the high quantity of powder necessary for a capsule of size less than or equal to 0. Indeed, for pharmaceutical products, it is unusual to use capsules of size greater than “0”, because of the difficulty to swallow large capsules [26]. This study aims to develop a formulation of conventional release tablets based on lyophilized aqueous extracts of Lannea microcarpa with as few excipients as possible using a simple process. It aims to provide practitioners and patients with an easy-to-use alternative treatment based on extracts of the plant.
Methodology
Materials
Plant material: The plant material consisted of trunk bark of Lannea microcarpa (Anacardiaceae), collected in the commune of Loumbila (Burkina Faso), a locality located 20 km from Ouagadougou. A sample of the plant was identified by a botanist of the Laboratory of Ecology of the Joseph KI-ZERBO University concerning the herbarium N° 1544 deposited at the Department of Forest Production of the National Center of Scientific and Technological Research (CNRST, Ouagadougou, Burkina Faso). The harvested bark was dried in a sunlight and dust-free environment and then pulverized with a Gladiator blade mill. The pulverized barks were subjected to an extraction by aqueous decoction and then lyophilized using the CHRIST® benchtop freeze dryer type ALPHA 1-2.
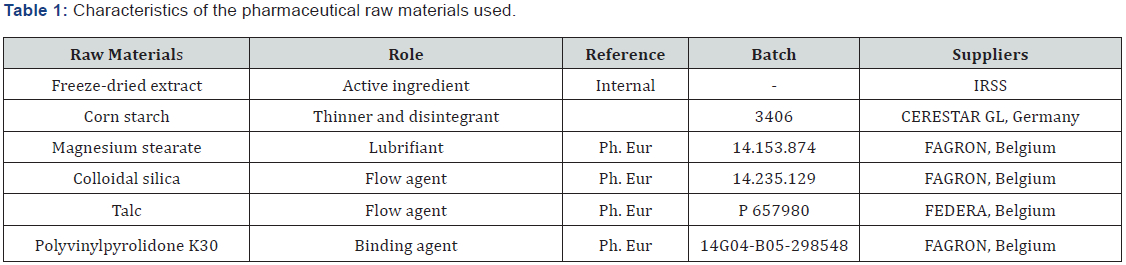
Excipients: Different types of excipients have been used and their characteristics studied to achieve an optimal formulation. The raw materials used, and their features shown in Table 1.
Methods
Characterization of lyophilisates
Residual moisture content: The residual moisture content of the extracts and the excipients were determined using the thermogravimetric method [27,28]. One (01) g of each powder is weighed in triplicate and placed in a previously tared watch glass in an infrared moisture Analyser (MF-50, US).
Hygroscopicity
Hygroscopicity was determined using 1.0 g of extract according to the method described in European Pharmacopoeia 6.0 [28]. The extract was introduced into a suitable desiccator containing a saturated ammonium chloride solution at 25°C for 24 hours. The percentage increase in mass was calculated according to the expression: ((m3-m2)/(m2-m1)) x100
m1: mass of the container
m2: mass of container + powder at T 0 hour
m3: mass of vessel + powder at T 24 hours
Solubility test
The solubility study was performed in distilled water. Solubility was determined by gradually adding increasing volumes. The maximum amount of substance required for this assay was 111 mg and the maximum solvent volume was 30 mL according to the European Pharmacopoeia 5th edition [21]. The mixtures were stirred vigorously with a magnetic stirrer for 1 minute and then placed in a thermostatically controlled chamber at a temperature of 25.0 ± 0.5 °C for 15 minutes.
Suitability for extract flow
The flowability of the extracts was realized through the determination of the compressibility index (carr index) and the Hausner index. The compressibility index (Carr’s index) and the Hausner index (Hausner’s index) were determined by measuring the apparent non-packed volume called bulk density and the tapped density after compacting the granules until a constant final volume. It was carried out with test piece according to the method described in European Pharmacopoeia 6.0 [29,30]. Both bulk density (BD) and tapped density (TD) were determined by pouring 10 g of granules from each formula into a 50 mL measuring test piece. The test piece was tapped three times onto a hard surface from the height of 2 cm at 2-second intervals. This volume was considered as a bulk volume. The tapping was continued until no further change in volume was noted.
This volume was considered as a tapped volume. BD and TD [30] were calculated using the following formulas:
Compressibility index or Carr’s index (%) = [(TD-BD) × 100] /TD [30]
BD = weight of the granule/volume of the packing
TD = weight of the granule/tapped volume of the packing
BD = weight of the granule/volume of the packing
TD = weight of the granule/tapped volume of the packing
Hausner’s ratio was determined using the following formula: Tapped density / Bulk density. A Hausner ratio greater than 1.25 is considered of poor flow ability [31,32].
Development of tablet formulas
a) Justification of the dosage form
This study aimed to develop conventional release tablets containing 250mg and 500mg of extract. The orientation of the galenic form was based on the one hand, on the physicochemical properties of the extract and the other hand on the chronic character of the disease, requiring ease of administration and conservation to improve the observance of the treatment. These characteristics were also determining factors in the choice of excipients and the process to be used to produce the tablets. The amount of extract per tablet was based on the work of Nitiema et al., [18]. Mixing tests of excipients alone without extracts and excipients with extracts have oriented the retained proportions. The wet granulation technique was used with freeze-dried aqueous extracts of Lannea microcarpa trunk bark powders as active substance according to the evaluated characteristics and properties.
b) Formulation strategy
The strategy was to develop formulations that meet the standards with as few excipients as possible for formulation development. Therefore, it was, necessary to evaluate the relevance and the possible need to use or restrict certain excipients in the formulation. Several excipients were used to study their impact on the formulations and to arrive at an optimal formulation. The wet granulation method was chosen because the properties evaluated were not optimal for direct compression. The granulation having improved the flow of powders, the influence of three (03) lubricants (magnesium stearate, colloidal silica, talc) has been studied.
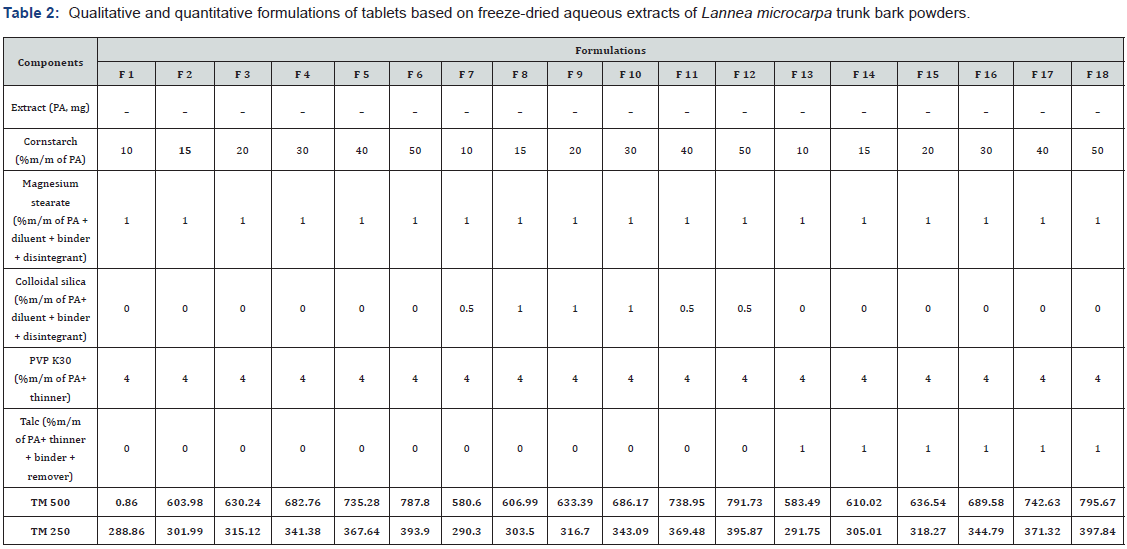
The first step was to make a series of formulations containing increasing amounts (10-50% w/w based on total extract weight) of diluent/disintegrant (corn starch), 4% w/w binding agent (PVP K30), and 1% w/w lubricant (magnesium stearate), corresponding to formulations F1 through F6 in Table II. The quantity of diluent was determined in these proportions of the theoretical mass of the tablet in order to obtain a homogeneous powder. In the second set of formulations (F7 to F12), 0.5% flow agent (colloidal silica) was incorporated into each of the initial formulations F1 to F6. Finally, in the third set of formulations (F13 to F18), colloidal silica was replaced with 1% w/w of another flow agent with antiadhesive properties, talc..
The theoretical mass was constituted by the mass of the internal phase associated with the mass of the external phase with a mass of internal phase constituted by the mass of the extract + half of the mass of diluent/disintegrant + the mass of binder. The mass of the external phase consisted of half the mass of the diluent/disintegrant + mass of lubricants.
c) Preparation of granules
The granulation was carried out with a Frewitt (Switzerland) oscillating pelletizer, equipped with an ERWEKA motor type AR 400 n°48581 (Switzerland). Ethanol at 96° was used as wetting liquid. Its volume varied from thirteen (13) for F1 to Twentyone (21) mL for F18. The mass to be granulated was weighed, mixed, and wetted with ethanol until a wet mass was obtained. This mass was introduced into the granulator and then subjected to an oscillatory movement forcing the paste to pass through the diameter sieves (1, 25mm). The obtained granules were dried in a Memmert oven (Germany) at a temperature of 45° C for 12 hours. The dried grains were sifted to retain grains of homogeneous size. The quality controls of the granules were carried out according to the official tests of the European Pharmacopoeia, namely, the macroscopic characteristics, the residual moisture content, the compressibility index and the Hausner index per methods 2.2.1.1, 2.2.1.2 and 2.2.1.3 [33].
d) Preparation of tablet
The mixtures of grains and lubricants were made in a Stephan® mixer in order to obtain a homogeneous product with a uniform distribution of the lubricant. The theoretical masses of the tablets were calculated, to compare them with the real masses obtained after compression. They were calculated according to the addition of the masses of the internal phases associated with the masses of the external phases. The tablets were manufactured with a manual rotary tablet press. Adjustments were made to the filling volume of the compression chamber and the compression force. The intervention on the volume of the compression chamber made it possible to obtain the mass of the tablet containing the calculated active dose of the extract. These adjustments made it possible to modulate the mass and hardness of the tablets to the desired values that meet the standards of the European Pharmacopoeia. Among the formulations made, the one meeting all the required qualities of the tablets were retained. The quality controls of the tablets were carried out according to the official tests of the European Pharmacopoeia, namely, the tests of mass uniformity, hardness, friability, disintegration time and chemical tracer content [33].
Pharmaceutical controls of tablets
Macroscopic characteristics: The macroscopic and organoleptic characteristics of the tablets obtained were described. It is about the appearance, the homogeneity of the colour, the taste and the smell of the tablets. Tablet dimensions such as diameter and thickness were also determined using a caliper [34,35].
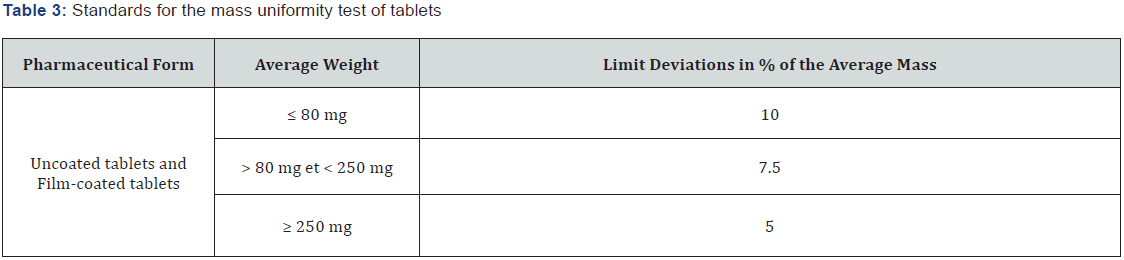
Mass uniformity test: This test was performed on twenty (20) tablets taken randomly from each batch and weighed individually with a precision balance (Sartorius, France). The average mass (M), the mass deviations, as a percentage [100 (mi - M)/M] from the average mass and the coefficient of variation (CV) were calculated. These parameters were compared to the limits specified in the European Pharmacopoeia 10th edition (2.9.5) [36], which states that the individual mass of not more than 2 of the 20 tablets may deviate from the average mass by more than the percentage given in Table 3 but the mass of no tablet may deviate by more than twice that percentage.
Hardness test: This test was carried out on 6 tables taken at random and introduced individually into the tubes of the disaggregation apparatus (Pharma test, France). A disc was place on each table and the disaggregation medium used was distilled water at 37°C. The time of complete disintegration of the first and last tablet was noted. (2.9.1) [36].
Disintegration time : This test was carried out on 6 tablets taken at random and introduced individually into the tubes of the disaggregation apparatus (Pharma test, France). A disc was placed on each tablet and the disaggregation medium used was distilled water at 37°C. The time of complete disintegration of the first and last tablet was noted. and the calculated average disintegration time. The results were compared to the limits specified in the European Pharmacopoeia 10th edition (2.9.1) [36].
Friability test : A sample of 20 tablets (m < 650 mg) and 10 tablets (m > 650 mg) was taken at random and placed in a friabilizer (Erweka, France) rotating at 25 rpm for 4 min. The tablets were weighed together before and after the test. The friability index determined must be less than 1%. The test was repeated 3 times according to the European Pharmacopoeia 10th edition (2.9.7) [36].
Determination of the uniformity of total phenolic compound content
A sample of 10 tablets was taken at random. Each tablet was weighed, pulverized with a mortar, and macerated individually for 10 min, by magnetic stirring, in a beaker containing 50 ml of distilled water. A 1 ml volume of the filtrate from each sample was prepared according to the method of Singleton et al and determined spectrophotometrically at 380 nm [37]. The content (%, m/m) of phenolic compounds in each tablet was determined by calculation, and the content of the powder used as raw material for tablet manufacturing. The test was repeated 3 times according to the European Pharmacopoeia 10th edition (2.9.6) [36].
Results and Discussion
Physicochemical characteristics of lyophilisates
The physicochemical characterization tests of the freezedried extracts showed that the residual moisture content was 4.39±0.15% (m/m) with a value (17.42±0.36% %) higher than 15 percent for the hygroscopicity test which indicates that the extract is very hygroscopic according to the European Pharmacopoeia 10. The solubility in water at 25°C of the extract was classified as easily soluble and those of the mixtures and granules were partially soluble according to the indications of the European pharmacopoeia 6.0. The flow properties realized by the tests of compressibility index (Carr’s index) was superior 38% and the Hausner index (Hausner’s index) superior at 1.6. This indicates according to the European Pharmacopoeia that the extracts have very poor flow properties. In contrast, the properties of powders to flow under given circumstances (fluidity) affects a large number of industrial applications [38]. One of the most advantageous processes for manufacturing of tablets is the direct compression of the active ingredient with suitable excipients [39,40]. However, for its realization, the active ingredients and excipients must demonstrate, good fluidity, compressibility and wettability [41]. Indeed, hygroscopicity plays an essential role in particle-particle interactions and can contribute to a poor fluidity of the powder and negatively affect the physical and chemical stability of the powder [42]. Previous studies have shown that freeze-dried extracts of Lannea microcarpa, like dry plant extracts in general, are complex materials that tend to be hygroscopic and sticky with poor physical and mechanical properties [43]. Therefore, the addition of suitable excipients and/or the use of appropriate processing technologies prior to compression is necessary. Thus, the amount of excipients that can be added becomes a critical step to manufacture tablets of reasonable size by compression [44,45]. Therefore, pre-treatment becomes almost mandatory to obtain dry plant extracts suitable for compression [46-48]. Granulation is then an alternative to this pre-treatment to improve the flow with fewer excipients.
Physicochemical characteristics of the granules
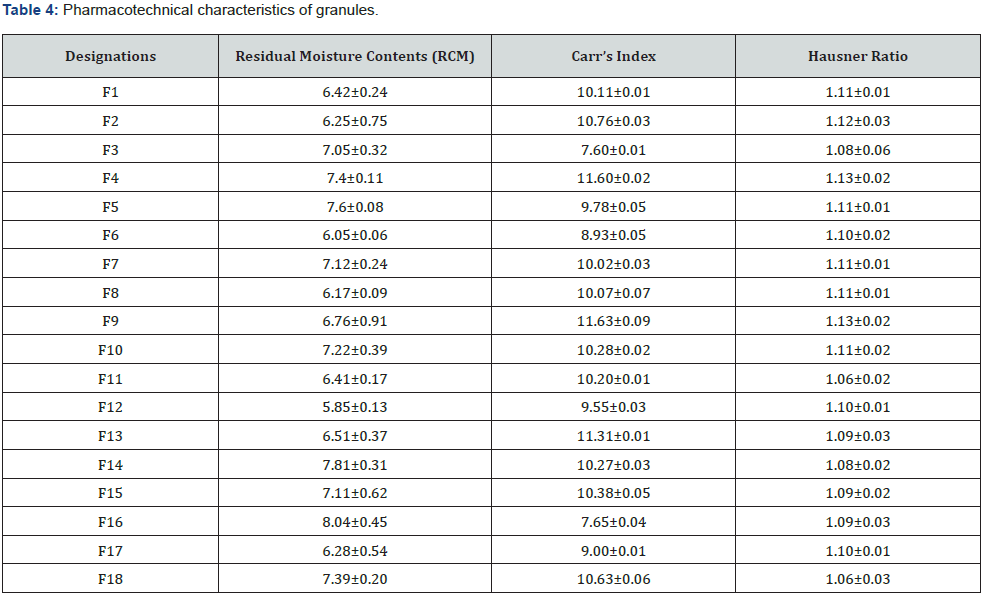
The physicochemical characterization tests of the Lannea Microcarpa based granules showed that granules were uniforms, brown in colour, with a weak, uncharacteristic odour and a slightly bitter taste (Figure 1). The granules had almost homogeneous grains because they were prepared using the same process with a sieve of the same mesh size. This is probably related to the fact that in the mixture some constituents, namely the excipients, are not soluble in water [49;50]. The residual moisture contents (RMC) are respectively 7.26±0.38% for the extracts and recorded in Table 4 for the granules formulations. All granules had a residual water content of less than 10%. This low moisture content could indicate better stability because it avoids possible enzymatic reactions and the development of microorganisms [51,52]. The compatibility tests performed by settling were classified into indices according to Table 4. They gave Hausner indices that ranged from 1.06±0.03 to 1.13±0.02 from F1 to F18, respectively, and Carr indices that ranged from 7.60±0.01 to 11.63±0.09 from F1 to F18. Therefore, all granules had good flow properties according to the European Pharmacopoeia. These indices are useful for characterizing the fluidity of granules and predicting their compressibility’s. Among parameters, flow of powders within these processes play a critical role in obtaining desirable characteristics of end products [53]. These analyses indicated that these granules could be used for compression.

Pharmacotechnical characteristics of the tablets
The results of mass uniformity, hardness and friability are presented in Tables 5 & 6.
The average masses of the tablets dosed at 250mg are recorded in Table 5 and varied from 263.187 mg ± 16.4 for FA1 to 379.979 ± 13.1 for FA18 with coefficients of variation between 2.72% and 6.23%. The average masses of the tablets dosed at 500mg are recorded in Table 6 and varied from 561.047mg ± 15.13 for FB1 to 783.388 ± 33.82 for FB18 with coefficients of variation ranging from 2.05% to 4.95%. As a percentage of the average mass, the deviations in individual tablet mass fell within limits allowed by the European Pharmacopoeia. These parameters make it possible to know if the tablets have the desired masses. The coefficients made it possible to measure the degree of dispersion of masses of the tablets around each average mass. Also, this parameter could be used in stability studies because any increase in mass during the storage period indicates absorption of moisture highlighting unsuitable conditions. The mass uniformity ensures that, the distribution of the powder mixture in the setting unit is uniform and sufficiently precise during the manufacturing process [54]. The analysis of mass variations shows that the 250 mg formulations had coefficients of variation that were relatively higher than those dosed at 500 mg. This indicates that the 500mg tablets had more homogeneous masses [55]. The mean hardness ranged from 24.9 N ± 0.61 to 57.32 N ± 2.35 for the 250 mg tablets and from 27.9 N ± 0.64 to 61.9 N ± 2.91. This variation could be related to the compatibility of each mixture but also a variation of the compression force. Indeed, during the use of a manual press, the compression force could be more or less important during each operation since development processes often focus on improving tablet characteristics such as hardness, disintegration time, stability and friability. This also provides an opportunity for the manipulator to set certain parameters to the desired characteristics. The understanding of the impact of the characteristics of the raw materials and the different parameters related to the manufacturing process allows to quickly solve the problems encountered, optimize the production of tablets and adapt to any modification (raw material, equipment, etc.) [56,57]. The analysis of the crumbling rates indicates that for the 250 mg tablet formulations, only the FA6, 11, 12, 17 and 18 tablets had crumbling rates within the norms according to the European Pharmacopoeia 10th edition (33). This friability rates (< 1%) of the tablets indicates that these tablets will have good shock resistance during storage and distribution operations (35,58). These crumbling rates of less than 1% indicate good resistance to crumbling and good cohesion of the particles during compression. It is related to the quality and quantity of the binding agent used (PVP) and or the variation of the compression force. This is made possible by pelletizing, which has a number of advantages, including improved particle compactibility, reduced dust emission and air entrapment during compression, and better homogenization of the mixture (59). Other studies confirm the improved mechanical performance of tablets by wet compression compared to conventional tablet forming routes (60, 61). For example, Bi et al., (60) show that lactose tablets are ten times stronger mechanically when made with the wet compression process than with conventional methods. In addition, the water added to the mixture to be compressed plays a key role because it allows the binder to dissolve and coat the particles in the mixture more easily. In addition, solid bridges are formed within the tablet during the drying period. These bridges allow the tablet to acquire a better mechanical strength than that obtained by other compression methods, where only Van der Waals forces and hydrogen bonds intervene to ensure the bonds between the particles, the solid bridges possibly present being created during the preceding stages (granulation in particular) (60,62). The disintegration times of the tablets took place in an acceptable conditions. All formulations had disintegration times of less than 15 minutes, although some had relatively high values close to 15 minutes. They were, therefore, in conformity with the requirements of the European Pharmacopoeia 10th edition. (Table 6). Into the overall analysis, the formulations had different disintegration times depending on their composition. This disintegration of all the tablets, lower than 15 minutes, was linked to the nature of the excipients, in particular corn starch, which leads to a sufficient disintegration in the proportions used. Indeed, corn starch is also used as disintegrating agents in order to accelerate the disintegration of the tablet, thus the release of the active ingredient in water and digestive juices. This suggests a relatively rapid release of the active substances contained in these tablets in the digestive tract (52).
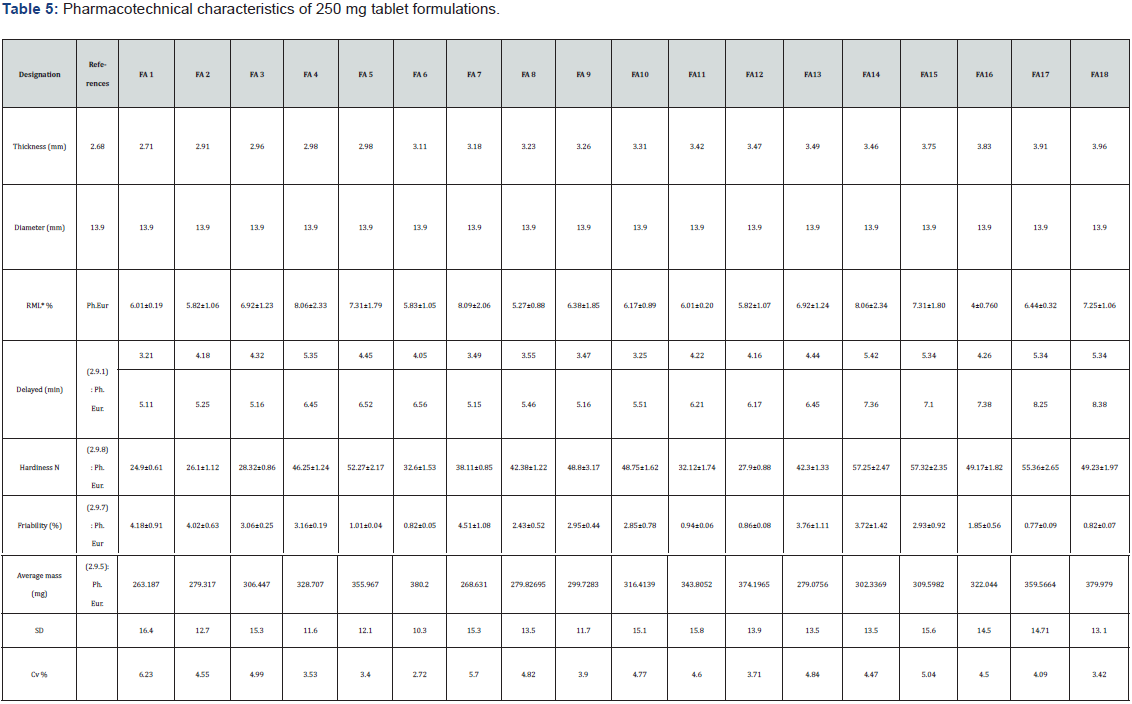
RML = Residual Moisture Level, SD = Standard deviation, Cv = Coefficient of variation

RML = Residual Moisture Level, SD = Standard deviation, Cv = Coefficient of variation
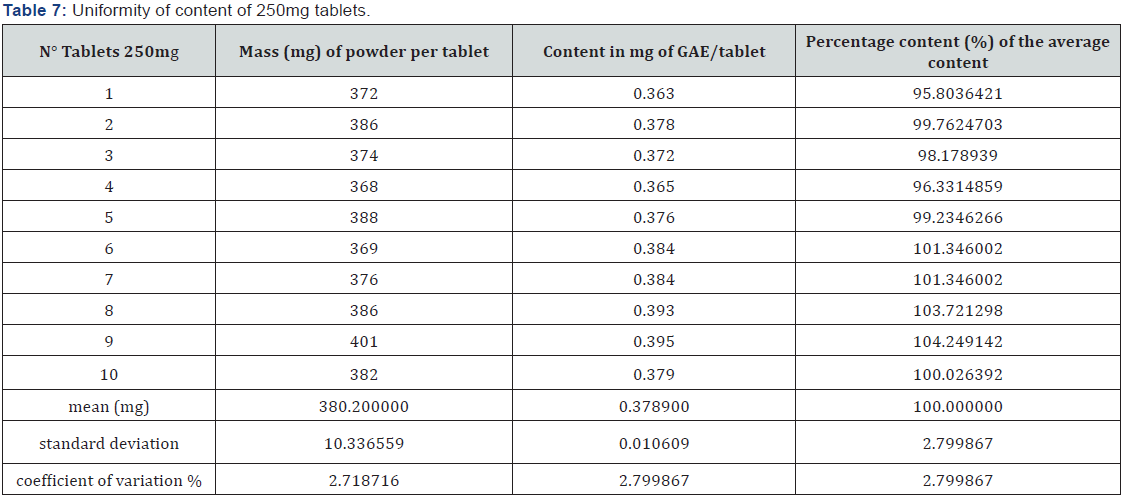
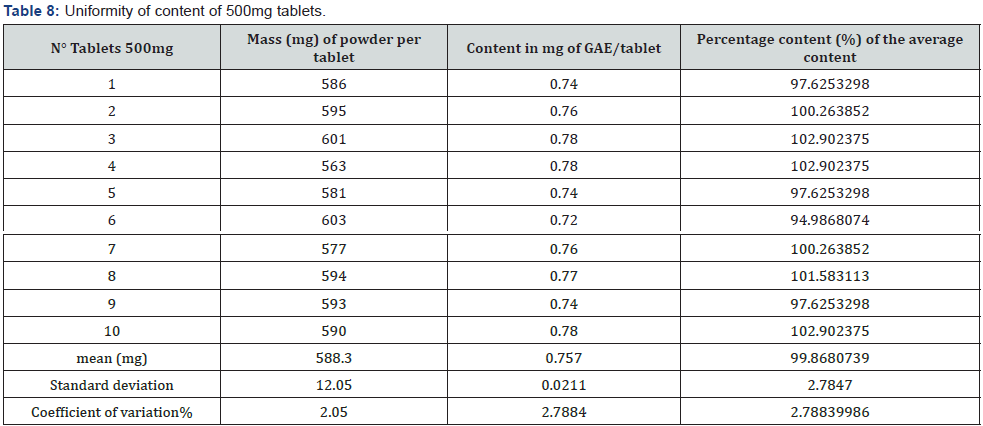

Determination of the content of phenolic compounds as a marker
The phenolic compound contents of the tablets were determined by calculation, from the calibration line whose equation is y = 10.459x + 0.0335 with a regression coefficient R2 = 0.9993. This resulted in an average content of 0.378900 ± 0.010609 mg gallic acid equivalent (GAE)/tablet and 0.757 ± 0.0211 mg GAE/tablet, respectively for the 250mg and 500mg tablets. From the content uniformity study represented by (Tables 7 & 8) the unique content of each tablet unit of both strengths is between 85 percent and 115 percent of the average content, which indicates that these tablets comply with the requirements of the European Pharmacopoeia. In effect, this pharmacopeia indicates that tablets fail the test if the individual content of more than one unit is not within these limits or if the unique content is outside the limits of 75 percent to 125 percent of the average content.
Conclusion
The present study was conducted to develop tablets for the treatment of hypertension. The formulation strategy used in this work allowed to realize mixtures of the active ingredient (lyophilized aqueous extract of Lannea microcarpa) with corn starch, PVP, colloidal silica, talc and magnesium stearate. The tablets were obtained by compression after wet granulation of the mixtures. The comparative analysis of the pharmacotechnical characteristics of the different formulations indicates that the FA6 and FB2 formulations showed the best properties such as disintegration, disintegration and hardness and meeting the requirements of the European Pharmacopoeia 10th edition. Additional studies such as the dissolution test and the stability study are necessary to conclude on the quality of the formulation. These pharmaceutical forms dosed at 250mg and 500mg in addition to being new will offer an alternative, because the use of mixtures in the form of powder and granules followed by a filling of capsules did not allow to obtain quickly a galenic form dosed at 500mg and meeting the recommendations of the pharmaceutical standards. This alternative in the management of the disease, allows reducing the production cost by compressed forms resulting from fast and straightforward manufacturing process with the least possible excipient and which do not use empty capsules.
Acknowledgement
African Center of Excellence for Training, Researches and Expertises on Drug (CEA-CFOREM) of World Bank for the rich collaboration.
Sources of Funding
FONRID (National Research and Innovation Fund for Development) for technical and financial support.
References
- Boinet T, Leroy-David C (2020) Hypertension artérielle essentielle chez l’ Actualités Pharmaceutiques 59: 13-7
- Damorou F, Pessinaba S, Tcherou T, Yayehd K, Ndassa S, et al. (2011) Hypertension artérielle du sujet noir âgé de 50 ans et plus à Lomé: aspects épidémiologiques et évaluation du risque cardiovasculaire (Etude prospective et longitudinale de 1485 patients). Proc. Annales de Cardiologie et d'Angéiologie 60: 61-6.
- Blacher J, Halimi JM, Hanon O, Mourad JJ, Pathak A, et al. (2013) Prise en charge de l’hypertension artérielle de l’ Recommandations 2013 de la Société française d’hypertension artérielle. La Presse Médicale 42: 819-825
- Pillon F, Michiels Y, Faure S (2014) Prise en charge de l’hypertension arté Actualités Pharmaceutiques 53: 25-29.
- Yaya S, Kengne A (2014) L’hypertension artérielle en Afrique: présent et nouvelles perspectives. uO Research.
- Rabi DM, McBrien KA, Sapir-Pichhadze R, Nakhla M, Ahmed SB, et al. (2020) Hypertension Canada’s 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Canadian Journal of Cardiology 36: 596-624.
- Diallo D, Guissou IP, Haidara M, Kasilo OM, Tall C (2010) Recherche sur la médecine traditionnelle africaine: hypertension. African Health Monitor 14: 58-63.
- OMS (2001) Promotion du rôle de la médecine traditionnelle dans le système de santé: Stratégie de la région africaine AFR/RC50/9, Harare: Bureau régional de l’Afrique p. 20.
- Musthaba M, Baboota S, Athar TM, Thajudeen KY, Ahmed S, et al. (2010) Patented herbal formulations and their therapeutic applications. Recent patents on drug delivery & formulation 4: 231-244.
- Qusaj Y, Leng A, Alshihabi F, Krasniqi B, Vandamme T (2012) Development strategies for herbal products reducing the influence of natural variance in dry mass on tableting properties and tablet characteristics. Pharmaceutics 4: 501-516
- Wu CY, Seville JPK (2009) A comparative study of compaction properties of binary and bilayer tablets. Powder Technology 189: 285-294.
- Kasilo OMJ, Wambebe C, Nikiema JB, Nabyonga Orem J (2019) Towards universal health coverage: advancing the development and use of traditional medicines in Africa. 4: e001517.
- Nacoulma O (1996) Plantes médicinales et pratiques médicales traditionnelles au Burkina-Faso: cas du plateau central. Plantes médicinales et pratiques médicales traditionnelles au Burkina Faso: Cas du plateau central 2: 320.
- Compaore S, Belemnaba L, Hounkpevi A, Idohou R, Zerbo I, et al. (2020) Diversity of plants used in the management of hypertension by three associations of traditional healers along a climate gradient in Burkina Faso. Advances in Traditional Medicine pp. 1-12
- Belemnaba L, Nitiema M, Traoré S, Somé N, Traore A, et al. (2014) Research of plants with antihypertensive potential in the biodiversity of Burkina Faso. Pharmacopoeia and traditional African medicine 17.
- Semde K, Dao MCE, Diallo BO, Ganaba S (2015) In the stands of Lannea microcarpa, are there individuals with different botanical characteristics in the Zorgho zone (Burkina Faso)? International Journal of Biological and Chemical Sciences 9: 2623-2632.
- Zague HW (2014) Etude des plantes a activite antihypertensive de la pharmacopee du Burkina Faso: evaluation in vitro de l'effet vasodilatateur de l'extrait aqueux des ecorces de troncs de lannea microcarpa engl. Et krause. (anacardiaceae).
- Nitiéma M, Soleti R, Koffi C, Belemnaba L, Mallegol P, et al. (2019) Ethyl Acetate Fraction of Lannea microcarpa Engl. and K. Krause (Anacardiaceae) Trunk Barks Corrects Angiotensin II-Induced Hypertension and Endothelial Dysfunction in Mice. Oxidative medicine and cellular longevity 2019: 14.
- Ouédraogo S, Belemnaba L, Zague H, Traore A, Lompo M, et al. (2010) Endothelium-independent vasorelaxation by extract and fractions from Lannea microcarpa Engl K. Krause (Anacardiaceae): possible involvement of phosphodiesterase inhibition. International Journal of Pharmacology and Biological Sciences 4: 9.
- Nitiéma M, Belemnaba L, Ouédraogo S, Ouédraogo N, Ouédraogo S, et al. (2018) Diuretic activity of aqueous decoction extract and ethyl acetate fraction of Lannea microcarpa engl. And k. Krause (anacardiaceae) trunk barks in wistar rats. World Journal of Pharmaceutical Research 7: 39-351.
- Belemnaba L, Soubeiga M, Ouédraogo GG, Traoré TK, Nitiéma M, et al. (2019) Antioxidant properties and subchronic toxicity of the standardized extract of LAMIC, a phytomedicine prototype based on aqueous extracts from trunk bark of Lannea microcarpa Engl and K. Krause. Journal of Drug Delivery and Therapeutics 9: 1-8.
- Owusu G, Antwi-Adjei M (2017) Acute and Sub-Acute Oral Toxicity Studies of the Aqueous Extract of Lannea macrocarpa Stem Bark on Rats. International Journal of Pharmacy and Pharmaceutical Research Human 9: 17-30.
- Ouédraogo S, Sombié BC, Ouédraogo JCW, Traoré TK, Traoré S, et al (2018) Standardization of Extracts from Trunks's Barks of Lannea microcarpa and K. Krause (Anacardiaceae) and Anogeissus leiocarpus (DC) Guill. and Perr. (Combretaceae) for the Formulation of Antihypertensive Herbal Medicines. Int. J. Pharm. Sci. Rev. Res 48: 92-97.
- Ouédraogo S, Sombié BC, Ouédraogo JCW, Nitiéma M, Belemnaba L, et al (2017) Quality control of trunk’s barks of Lannea microcarpa Engl. and K. Krause and Anogeissus leiocarpus (DC) Guill. & Perr. for the manufacture of phytomedicines for the treatment of hypertension. International Journal of Phytopharmacy 7: 36-41.
- Ouédraogo S (2016) Formulation galénique et contrôle de qualité des extraits des écorces des troncs de Lannea microcarpa and K. Krause (Anacardiaceae) et de Anogeissus Leiocarpus (DC) Guill. et Perr (Combretaceae) destinés à la prise en charge de lhypertension artérielle. Université Ouaga I Joseph Ki-Zerbo p. 106.
- Ridgway K, Cole GC, Jones BE, Jones RT, Newton JM (1987) Hard capsules: development and technology. pp 320. Pharmaceutical Press. P. 93.
- Eur (2011) European Pharmacopoeia: Technical Guide for the elaboration of monographs. European Directorate for the Quality of Medicines & HealthCare 6th Edition.
- Eur (2008) European Directorate for the Quality of Medicines and HealthCare (EDQM) of the Council of Europe. European Pharmacopoeia. General chapter Characters section in monographs 6th Edition tome 2 Strasbourg, France, pp. 2384.
- Ranpura VD, Kathiriya A, Shah KV (2013) Formulation, development and characterization of sustained release bilayered tablet of Valsartan and Pioglitazone HCl Pharma Science Monitor.
- Mahajan P, Mahajan S, Mishra D (2011) Valsartan release from sustained release matrix tablet and effect of cellulose derivatives. Int J Pharm Sci 2: 521-530.
- Deepak P, Abhishek J, Rakesh K, Hariharanand S (2011) Formulation and evaluation of pioglitazone hydrochloride matrix tablet containing aloe barbadensis miller mucilage natural antidiabetic agent. International Journal of Drug Discovery and Herbal Research 1: 157-163.
- Eur (2008) Pharmacopée Européenne, 6ème édition, conseil d'Europe. Stratbourg.
- Eur (2019) Pharmacopée européenne 10ème édition conseil de l'europe.
- Goyanes A, Buanz AB, Hatton GB, Gaisford S, Basit AW (2015) 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. European Journal of Pharmaceutics and Biopharmaceutics 89:157-162.
- N’Guessan A, Any-Grah SA, Dally IL, Tuo A, Lia AG, et al. (2019) Formulation de comprimés à base de feuilles et de tiges dArtemisia annua.
- Eur (2019) Pharmacopée Européenne (10th édn.), pp. 1-379.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in enzymology 299: 152-178.
- Santomaso A, Lazzaro P, Canu P (2003) Powder flowability and density ratios: the impact of granules packing. Chemical Engineering Science 58: 2857-2874.
- Lieberman HA, Lachman L, Schwartz JB (1980) Pharmaceutical dosage forms: Tablets. M. Dekker
- Jivraj M, Martini LG, Thomson CM. 2000. An overview of the different excipients useful for the direct compression of tablets. Pharmaceutical Science & Technology Today 3: 58-63.
- Gallo L, Ramírez-Rigo MV, Piña J, Palma S, Allemandi D, Bucalá V (2012) Valeriana officinalis Dry Plant Extract for Direct Compression: Preparation and Characterization. Scientia pharmaceutica 80: 1013-1026.
- Jin P, Madieh S, Augsburger LL (2008) Selected physical and chemical properties of Feverfew (Tanacetum parthenium) extracts important for formulated product quality and performance. AAPS PharmSciTech 9: 22-30.
- Tong HH, Wong SY, Law MW, Chu KK, Chow AH (2008) Anti-hygroscopic effect of dextrans in herbal formulations. Int J Pharm 363: 99-105.
- Rocksloh K, Rapp FR, Abu Abed S, Müller W, Reher M, et al. (1999) Optimization of crushing strength and disintegration time of a high dose plant extract tablet by neural networks. Drug development and industrial pharmacy 25:1015-1025.
- De Souza TP, Bassani VL, González Ortega G, dalla Costa TC, Petrovick PR (2001) Influence of adjuvants on the dissolution profile of tablets containing high doses of spray-dried extract of Maytenus ilicifolia. Die Pharmazie 56: 730-733.
- Palma S, Luján C, Llabot JM, Barboza G, Manzo RH, et al. (2002) Design of Peumus boldus tablets by direct compression using a novel dry plant extract. Int J Pharm 233: 191-198.
- Soares LAL, González Ortega G, Petrovick PR, Schmidt PC (2005) Dry granulation and compression of spray-dried plant extracts. AAPS PharmSciTech 6(3): E359-E66.
- De Souza TP, Martínez-Pacheco R, Gómez-Amoza JL, Petrovick PR (2007) Eudragit E as excipient for production of granules and tablets from Phyllanthus niruri L spray-dried extract. AAPS Pharm Sci Tech 8: E54-E60.
- Ariyasu A, Hattori Y, Otsuka M (2016) Delay effect of magnesium stearate on tablet dissolution in acidic medium. International journal of pharmaceutics 511: 757-764.
- Jadhav N, Paradkar A, Salunkhe N, Karade R, Mane G (2013) Talc: A versatile pharmaceutical excipient. World Journal of Pharmacy and Pharmacutical Sciences 2: 4639-46360
- Dawoodbhai S, Rhodes CT (1989) The effect of moisture on powder flow and on compaction and physical stability of tablets. Drug development and industrial pharmacy 15:1577-1600.
- Chowhan Z (1979) Moisture, Hardness, Disintegration and Dissolution Interrelationships in Compressed Tablets Prepared by the Wet Granulation Process. Drug development and industrial pharmacy 5: 41-62.
- Kaleem MA, Alam MZ, Khan M, Jaffery SHI, Rashid B (2020) An experimental investigation on accuracy of Hausner Ratio and Carr Index of powders in additive manufacturing processes. Metal Powder Report.
- Hegedűs Á, Pintye Hódi K (2007) Comparison of the effects of different drying techniques on properties of granules and tablets made on a production scale. International journal of pharmaceutics 330: 99-104.
- Madathilethu J, Roberts M, Peak M, Blair J, Prescott R, Ford JL (2018) Content uniformity of quartered hydrocortisone tablets in comparison with mini-tablets for paediatric dosing. BMJ paediatrics open: 2.
- Brisson J, Genin M, Tabib N (2006) Les défauts de fabrication des comprimé Pharmaterm MD 17: 1-6
- Geoffroy JM, Rivkees D (2008) Pharmaceutical manufacturing: changes in paradigms. Pharmaceutical Dosage Forms-Tablets: Manufacture and Process Control, (3rd ), pp. 85-119.
- Osei-Yeboah F, Sun CC (2015) Validation and applications of an expedited tablet friability method. International Journal of Pharmaceutics 484: 146-55.






























