The Co-Mutation of CDKN2A And PDCD1LG2 May be A Predictor of Duvalizumab -Induced Cytokine Storm in Non-Small-Cell Lung Cancer: A Case Report
Yonglong Jin1, Yinshi Cui2, Wenjing Xiao1, Bo Li1, Jinpeng Xu1, Dunmin Zhuang1, Lina Dong1 and Xiguang Liu1*
1Department of Radiotherapy, Affiliated Hospital of Qingdao University, China
2Department of Cardiology, Qingdao Fuwai Cardiovascular Hospital, China
Submission: July 29, 2021; Published: September 13, 2021
*Corresponding author: Xiguang Liu, Department of Radiotherapy, Affiliated Hospital of Qingdao University, China
How to cite this article:Yonglong J, Yinshi C, Wenjing X, Bo L, Jinpeng X, et al. The Co-Mutation of CDKN2A And PDCD1LG2 May be A Predictor of Duvalizumab -Induced Cytokine Storm in Non-Small-Cell Lung Cancer: A Case Report. Glob J Pharmaceu Sci. 2021; 8(5): 555749. DOI: 10.19080/GJPPS.2021.08.555749.
Abstract
In recent years, evidence has accumulated that neoadjuvant immunotherapy may improve the long-term survival of patients with respectable Non-Small Cell Lung Cancer (NSCLC). Some positive biomarkers that can predict efficacy have been clinically applied to screen patients subjected to immunotherapy. However, the negative biomarkers such as those associated with Immunotherapy-related cytokine storm remain elusive. Here, we report a case of elderly squamous cell NSCLC in stage IIA with high PD-L1 expression. The patient received 2 cycles of neoadjuvant therapy with duvalizumab combined with chemotherapy and then underwent surgical resection, reaching PCR after operation. Soon, the patient developed immune related adverse events and eventually died of multiple organ failure with only 2.5 months of the time to death. Cytokine detection indicated that the expression of multiple cytokines was increased, which was considered to be a cytokine storm caused by immunotherapy. Postoperative specimens were sequenced by Next Generation Sequencing (NGS), finding a Co-mutation of CDKN2A and PDCD1LG2. Combined with previous studies and the changes of cytokine level and gene expression profile of the patient, we consider that the co-mutation of CDKN2A and PDCD1LG2 is closely related to cytokine storm, and it may be a negative biomarker of immune checkpoint inhibitors.
Keywords: Cytokine storm; CDKN2A; NSCLC; PDCD1LG2
Abbreviations: NSCLC: Non-Small Cell Lung Cancer; NGS: Next Generation Sequencing; ICIs: Immune Checkpoint Inhibitors; CTLA-4: Cytotoxic T Lymphocyte Associated Antigen-4; PD-1: Programmed Cell Death Protein; PD-L1: Programmed Death Ligand-1; IRAEs: Immune-Related Adverse Events; EGFR: Epidermal Growth Factor Receptor; CT: Computed Tomography; ALK: Anaplastic Lymphoma Kinase; NSE: Neuron-Specific Enolase; PCR: Pathologic Complete Response; CRRT: Continuous Renal Replacement Therapy; MODS: Multiple Organ Dysfunction Syndrome
Introduction
Different from surgery, chemotherapy and radiotherapy, Immune Checkpoint Inhibitors (ICIs) reactivate the immune response effect of T cells by inhibiting the activity of Programmed Cell Death Protein 1 (PD-1), Programmed Death Ligand-1 (PD-L1), and Cytotoxic T Lymphocyte Associated Antigen-4 (CTLA-4), thereby repressing tumors [1]. The increasing number of studies has demonstrated that PD-L1 inhibitor-based neoadjuvant immunotherapy is beneficial in respectable Non-Small Cell Lung Cancer (NSCLC) [2]. However, in practical application neoadjuvant immunotherapy has been faced with many challenges such as ICIs-Induced Immune-Related Adverse Events (IRAEs). According to a study, 31,059 patients with ICIs-related diseases were retrieved out of 16 million patients by searching the global adverse drug reaction database Vigilyze-Vigibase - of these, 613 had fatal IRAEs [3]. Currently, no reliable biomarkers have been identified in predicting IRAEs due to a lack of the underlying mechanistic knowledge and treatment experience, therefore, IRAEs in NSCLC require further investigation. In the present study, a patient with squamous cell NSCLC suffered from a Co-Mutation of CDKN2A and PDCD1LG2 and ultimately died from a cytokine storm after treatment with ICIs.
Case Presentation
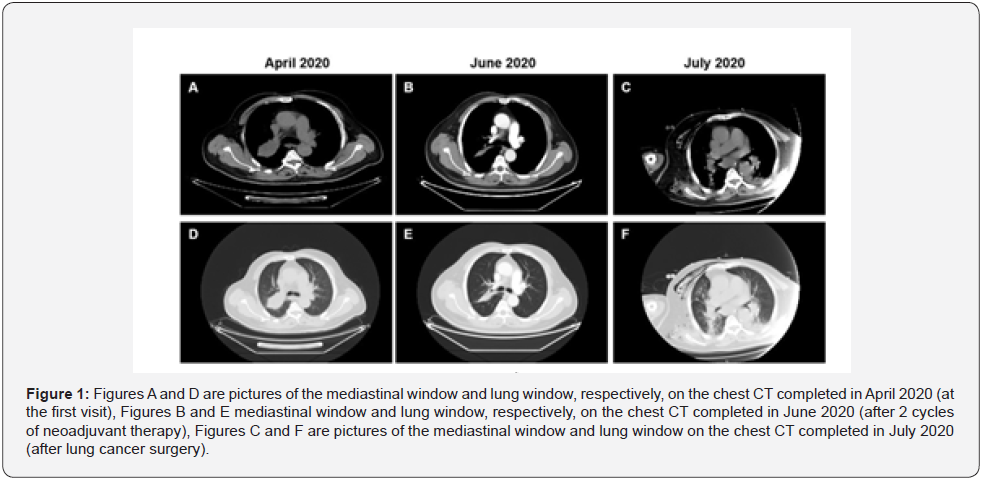
A 65-year-old, non-smoking male patient, presented to the Department of Oncology of our hospital with a 1 month history of cough on April 12, 2020. The positron emission Computed Tomography (CT) showed a mass in the upper lobar bronchus of the right lung and obstructive pulmonary atelectasis but no lymph node metastasis in the mediastinum (Figure 1). To identify the lesion nature, a CT guided biopsy of the right upper lobar mass was performed on April 20, 2020, and the pathological results suggested squamous cell cancer (non-keratinization/moderate differentiation). The genetic analysis of the tissue sample showed no mutation of driver genes such as Epidermal Growth Factor Receptor (EGFR), Anaplastic Lymphoma Kinase (ALK), C-ROS Oncogene 1 (ROS1), as well as a 70% positive PD-L1 expression (IHC 22C3 pharmDx) with a lesion stage of IIA cT2bN0M0 (the Union for International Cancer Control and the American Joint Committee on Cancer, the 8th edition). Upon multidisciplinary consultation, the patient decided to receive a neoadjuvant therapy. A total of two cycles of neoadjuvant therapy was performed lasting from May 8, 20 to June 1, 2020, specifically with Albumin- Bound paclitaxel at 200 mg q3w on d 1, d 8, and d 15, carboplatin at 600 mg q3w on d 1 and duvalizumab at 1,000 mg q2w. After the second cycle of neoadjuvant therapy, a CT reexamination of the chest displayed that the upper lobar lesion in the right lung basically disappeared with obviously alleviated atelectasis. The levels of tumor markers such as Carcinoembryonic Antigen (CEA), Squamous Cell Carcinoma antigen (SCC), cytokeratin 19 fragment antigen 21-1(CYFRA21-1), and Neuron-Specific Enolase (NSE) declined, with CEA decreasing most prominently (about 50% decrease). The patient was referred to the Department of Thoracic Surgery after the second multidisciplinary consultation and the examination of liver, kidney and thyroid functions showed no abnormalities. On July 7, 2020, he underwent videoassisted thoracoscopic right upper lobectomy and systemic lymph node dissection under general anesthesia. The postoperative pathological results indicated neither malignant tumor cells in the endoscopic lung tissue and at the tracheal resection margin nor tumor metastasis in the regional lymph nodes, including lymph nodes surrounding the bronchus (0/2), group 2 lymph nodes (0/6), group 4 lymph nodes (0/4), group 7 lymph nodes (0/2), group 10 lymph nodes (0/1), group 11 lymph nodes (0/1), and group 12 lymph nodes (0/3). The postoperative specimens suggested Pathologic Complete Response (PCR) in the patient after neoadjuvant therapy. Two days post-surgery, he suddenly experienced chest distress and could not lie on his back. According to the vital signs monitoring, the patient experienced tachycardia (120-155 beats/min), had an increased blood pressure (210/130 mmHg) and fever (41℃), and wet rales were noticeable in both lungs as shown by auscultation. The chief, resident in the Department of Large Internal Medicine, made a consultation and considered that the patient suffered from acute heart failure. After diuresis, coronary artery dilation, and ventricular rhythm stabilization treatment, no improvement was observed so that he was referred to the intensive care unit (ICU). In the ICU, the patient had persistent hyperpyrexia (40-43℃) with increased C-reactive protein levels (34.8 mg/L) and the CT of the chest showed inflammation in both lungs.
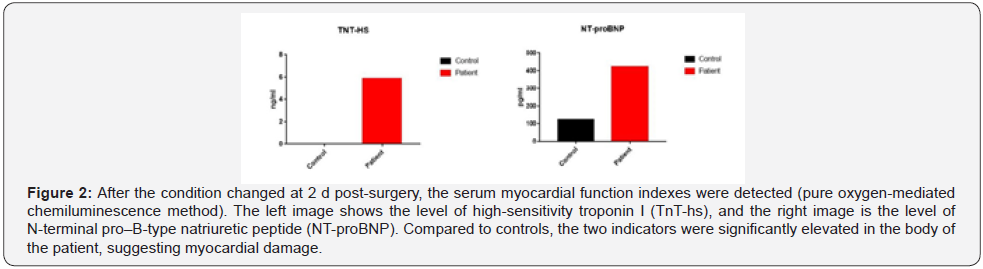
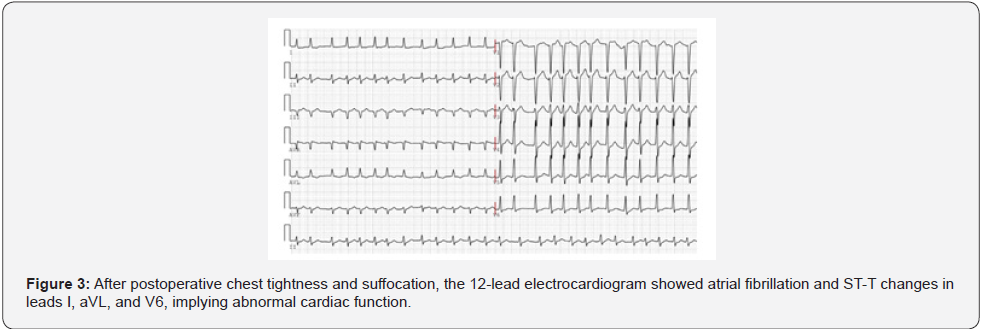
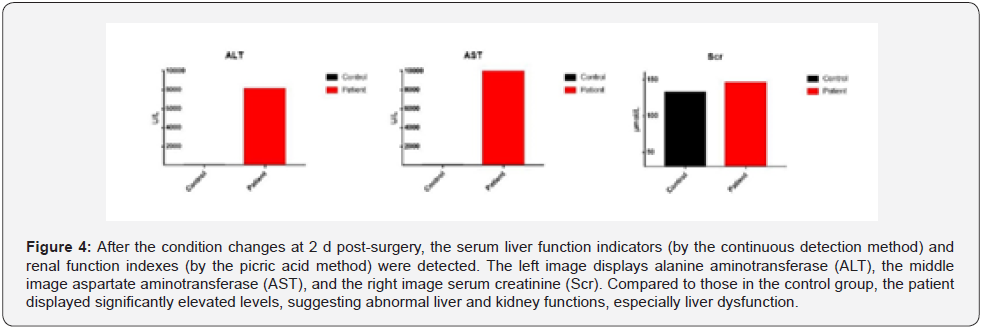
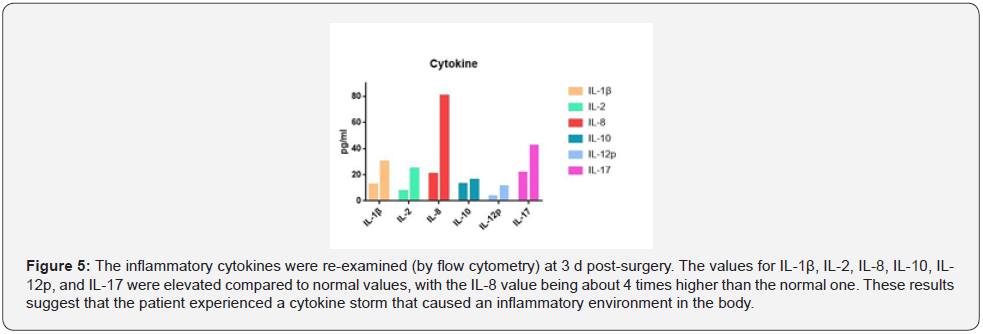
Upon approval by the Pharmacy Group and Antibacterial Drug Management Group of the hospital, cefoperazone sulbactam and linezolid were administered for Anti-Infection treatment. High-Sensitivity troponin T (5.889 ng/mL) and N-Terminal B-Type natriuretic peptide precursor levels (423 pg/mL) were elevated (Figure 2) and the electrocardiogram showed atrial fibrillation with Multi-Lead ST-T changes (Figure 3). Due to these findings, the patient was suspected of having a non-ST segment elevation myocardial infarction which was dealt with by supportive treatments, including antiplatelet treatment, Anti-Coagulation treatment and coronary artery dilation. In addition to the above-mentioned parameters, the levels of blood alanine aminotransferase (ALT, 8,100 U/L), aspartate (AST, 10,300 U/L), and creatinine (146 μmol/L) were raised (Figure 4). The Department of Oncology was invited for consultation which resulted in the diagnosis that the patient was considered to suffer from Immune-Related myocardial injury, pneumonia, hepatitis and nephritis. The Re-Test of cytokines indicated increases in the levels of interleukin (IL)-1β, IL-2, IL-8, IL-10, IL- 12p, and IL-17 compared to those prior to neoadjuvant therapy. Of these, IL-8 showed the most dramatic effect by increasing fourfold (Figure 5). Large panel genetic tests can detect gene mutations, amplifications, fusion and other aberrations obviously related to clinically relevant tumors. A retrospective genetic test was conducted on the specimens using the Next Generation Sequencing (NGS) technique to pinpoint the cause of the cytokine storm and it was revealed that CDKN2A and PDCD1LG2 were mutated with a tumor mutation burden (TMB) of 14.53 Muts/ Mb. The supportive treatments were continued in the ICU with hormone and Anti-Immune treatments. The disease was gradually exacerbating instead of improving. One-week post-surgery, infections by Klebsiella pneumoniae and Citrobacter were found by a bacterial sputum culture without excluding fungal infection. After a review of the Pharmacy Group and the Antibacterial Drug Management Group of the hospital, the antibiotics were replaced with imipenem, teicoplanin, and compounding acetate for anti-infection treatment. Two weeks post-Surgery, the patient suffered from hypernatremia that was remediated by Continuous Renal Replacement Therapy (CRRT). Vascular ultrasonography indicated thrombosis in multiple veins, including the brachial vein, femoral vein, peroneal vein, and intermuscular vein. Anti- Coagulation and anti-platelet treatments were conducted, and the number of platelets was dynamically monitored. If necessary,platelet transfusion therapy was performed. Three weeks postsurgery, the disease further worsened with the patient entering into a shock and requiring a large dose of vasoactive agents for maintaining the blood pressure. In parallel, severe metabolic acidosis and liver failure developed. Since the number of accompanying families was limited to 1 during the COVID-19 pandemic, the patient’s family members asked for discharge and hoped the patient would go back home. As a result, the discharging formalities were completed on the basis of the principles of hospice care. During follow up, it turned out that the patient died at home 1 d after discharge. In this case, the patient had a stage IIA squamous cell NSCLC with 70% positive PD-L1 expression at the initial diagnosis and treatment. Therefore, a decision was made to perform 2 cycles of neoadjuvant therapy lasting from May 2020 to June 2020 with albumin-bound paclitaxel at 200 mg q3w on d 1, d 8, and d 15, carboplatin at 600 mg q3w on d 1 and duvalizumab at 1,000 mg q2w. After this, surgical treatment was completed in July 2020. After surgery, the patient had complete remission, achieving pCR. However, the patient then experienced symptoms of chest distress associated with suffocation, followed by immune-related myocardial injury, pneumonia, hepatitis, and nephritis, and ultimately died from Multiple Organ Dysfunction Syndrome (MODS) with a Time to Death (TTD) of only 2.5 months. The postoperative cytokine test indicated that the expression of multiple cytokines was increased, leading to a reasonable suspicion of a cytokine storm after immunotherapy. The further genetic test results demonstrated that the cytokine storm might be correlated to the co-mutation of CDKN2A and PDCD1LG2.
Discussion
Lung cancer ranks top 1 among the malignant tumors worldwide in terms of incidence and death rates, 85% of which is NSCLC [4]. Despite surgical treatment, the risk of postoperative recurrence and metastasis rose from 15% at stage IA to 60 % at stage IIIA, while the 5-year survival rate declined from 67% to below 25% in NSCLC patients [5]. Therefore, it is of fundamental importance to explore the optimal treatment schemes for operable NSCLC. Neoadjuvant immunotherapy blocks the signal transduction between the immunosuppressive molecule PD-L1 on the surface of tumor cells and the PD-1 receptor on the surface of immune cells mainly through competitively binding to PDL1 on the surface of tumor cells and in turn reactivates T cells, thereby exerting the effect of immune surveillance. The possibility of neoadjuvant immunotherapy has been identified from the immune mechanism and principle in Neoadjuvant PD-1 Blockade in Resectable Lung Cancer, a clinical trial on the ICI Nivolumab (Nivo) for neoadjuvant immunotherapy in operable NSCLC, whose results were firstly released in the American Association for Cancer Research Annual Meeting 2018 and simultaneously published in the New England Journal of Medicine in April 2018 [6]. Neoadjuvant chemotherapy has positive implications in eliminating residual micro metastases, reduction of tumor burden, and increase in surgical resection rate. Compared to neoadjuvant chemotherapy alone, the combined neoadjuvant immune chemotherapy, as an emerging treatment mode, tends to have more obvious survival benefits, thereby producing a better treatment efficacy [7]. The mechanism is mainly due to the toxicity of chemotherapeutic drugs that can stimulate tumor cell mutations, leading to the release of tumor neoantigens, thereby finally activating the antitumor immune response in the body, and enhancing the sensitivity of ICIs [8]. On the other hand, chemotherapeutic drugs can induce the normalization of tumor blood vessels, increase the infiltration by dendritic cells and clonal amplification of effector T cells in the microenvironment, thereby reducing the number of regulatory T cells and reconstructing the immune microenvironment [9]. Neoadjuvant chemotherapy and Nivo in respectable non-small cell lung cancer was a Multi-Center clinical trial to explore the efficacy of immunotherapy combined with chemotherapy in patients with respectable stage IIIa NSCLC [10]. Nivo, paclitaxel and carboplatin (3 weeks) were administered preoperatively in the experiment group and surgery was conducted at 3-4 weeks after neoadjuvant therapy, followed by one year of persistent Nivo treatment. As of May 2019, 46 patients were enrolled in the study, 83% of which achieved major pathologic response (MPR), 71% pCR and 93% downstaging according to the results. Inspired by the study, multiple phase III clinical trials (IMpower 030, KEYNOTE-671,CheckMate 816, and CheckMate 77T) are under progress to further reveal the clinical efficacy of the mode of neoadjuvant immunotherapy + chemotherapy and its status in the future of neoadjuvant therapies. In NCT02716038, a Single-arm phase II clinical trial, preoperative neoadjuvant therapy was conducted with carboplatin + Albumin-Bound paclitaxel + MPDL3280A (PDL1 antibody) in the experiment group [11]. According to the phase I results, published in the American Society of Clinical Oncology in 2018, of the enrolled 14 cases of respectable NSCLC, 50% had MPR, 21.4% pCR, 85.7% grade 3-4 adverse reactions and 64.3% a decline in chemotherapy agent doses due to adverse reactions. These data suggest the negative side effects of the mode of neoadjuvant immunotherapy + chemotherapy. Taken together, a large proportion of patients achieve MPR following preoperative combination of immunotherapy and chemotherapy in clinical practice, quite a part of which experienced complete remission of tumors and lymph nodes (i.e. ypT0N0), who may survive for a longer time period. The combined immunotherapy can produce higher MPR and pCR rates than neoadjuvant chemotherapy alone. However, much attention should be paid to the severity of treatment side effects. Currently, there is no definite guideline and recommendation for the dose and interval of preoperative neoadjuvant immunotherapy, but several major drugs have their common doses. Normally, the interval for two consecutive treatment cycles with Nivo, duvalizumab and carrelizumab was two weeks, while the interval was three weeks for the treatment with pembrolizumab, atezolizumab and sintilizumab [12]. In terms of medication duration, 2-4 cycles are chosen after comprehensive consideration of various factors such as efficacy, timing of surgery, patient compliance, economic conditions, etc. In most studies, however, a higher level of clinical evidence is required to determine the optimal medication regimen [13]. In the present study, a case of stage IIA squamous cell NSCLC was reported, and the neoadjuvant therapy was conducted with PD-L1 inhibitor and chemotherapeutic agents for two cycles. The patient achieved pCR after surgery. However, unfortunately, he suffered from Immune related myocardial injury, pneumonia, hepatitis and nephritis after operation, suspected of having experienced a cytokine storm, and ultimately died from MODS with a TTD of 2.5 months.
At present, biomarkers such as PD-L1, TMB, and deficient mismatch repair (dMMR) have been considered as predictors for the evaluation of PD-1/PD-L1 inhibitor efficacy [14]. In our preliminary work, one patient with stage IV lung squamous cell carcinoma was admitted with malignant pleural effusion, and iliac bone, liver, and other body metastases. No mutations were found in EGFR, ALK, ROS1 and other driver genes, so platinum containing dual drug chemotherapy with albumin paclitaxel + carboplatin was used for first-line treatment, followed by disease progression. The histopathological examination and genetic test of the discharged pleural effusion showed that the expression rate of PD-L1 was 50% with a TMB of 27.42 Muts/Mb. As recommended by the National Comprehensive Cancer Network guidelines, PD-L1 inhibitor therapy was chosen combined with external radiation therapy for the right ilium metastases. After 6 cycles of immune therapy and radiotherapy for the right iliac metastases, the lesions basically disappeared, which was assessed as CR according to the Response Evaluation Criteria in Solid Tumors (v1.1) and remain stable now, inferring that the patient benefited from the high expressions of PD-L1 and TMB. Nevertheless, ICIs can sometimes cause a cytokine storm, hyper progression and other severe IRAEs, even Life-Threatening ones. In the largest evaluative study of fatal side effects ever, published in the JAMA Oncology, the 3,545 cases of data from seven centers suggested that the mortality rate is 0.6% among the patients receiving ICIs therapy, however, especially neoadjuvant therapy and severe side effects may cause delayed surgery and even fatal consequences [15]. While the benefits in clinical application are obtained, the problem of side effects should be decreased to the largest extent possible. Thus far, no definite negative biomarkers have been found to predict the occurrence of IRAEs. Therefore, a full evaluation verifying the presence or absence of related adverse, or risk factors should be made prior to ICIs treatment. In this case, PD-L1 was highly expressed, and tumors obviously shrunk after ICIs therapy combined with chemotherapy which was indicated as pCR as shown by the postoperative specimens. However, the disease was worsened after surgery and the expression of cytokines significantly rose compared to those prior to surgery, which manifested in a cytokine storm. Genetic analysis revealed that both CDKN2A and PDCD1LG2 were mutated. Although proactive symptomatic and supportive treatments were given later, the disease condition could not be reversed, and the patient died from MODS. CDKN2A, identified by Serrano in the 1990s, is located on human chromosome 9, band p21, with a full length of 8,500 bp containing 3 exons. It encodes 2 cell cycle inhibitory proteins, p16INK4a and p14ARF, that regulate the cell cycle [16]. The tumor suppressor gene CDKN2A binds to CDK4 and CDK6, inhibits the formation of Kinase-Active complex of cyclin and CDK4, thereby blocking the phosphorylation of retinoblastoma protein by such a complex, causing a cell cycle arrest in the G phase and ultimately inhibiting cell proliferation. CDKN2A homozygous deletion, promoter methylation or gene locus mutation can lead to the loss of p16 and p14 expression which is related to poor prognosis in NSCLC patients [17]. The PDCD1LG2 gene encodes PD-L2 which belongs to the ligands of PD-1, is located on chromosome 9, band p24, and serves as a member of the B7 superfamily [18]. PD-L2, a newly found negative co-stimulatory molecule, serves as the surface marker to induce apoptosis of activated T cells and binds to the IgV in its receptor PD-1. The resulting signals are transduced to the immunoreceptor tyrosine-based inhibitory motif in the cytoplasmic tail of PD-1, thereby playing an important negative regulatory role in immune response.
In recent years, some studies have found that PD-L2 is highly expressed in patients with rheumatoid arthritis and coronary atherosclerotic heart disease and it is believed to modulate the inflammatory environment probably by adjusting the functional state of T cells in local tissues [19]. The present case may be the first case of squamous cell NSCLC with high expression of PD-L1 and co-mutation of CDKN2A and PDCD1LG2. After neoadjuvant therapy, although the efficacy reached pCR, immediately a cytokine storm appeared. Based on the above study reports combined with the changes in cytokines and gene expression profiles, it was considered that the Co-Mutation of CDKNA and PDCD1LG2 was closely correlated to the cytokine storm. This may lay a foundation for the evaluation of CDKNA and PDCD1LG2 as negative biomarkers of ICIs application. The specific mechanism by which the co-mutation of CDKN2A and PDCD1LG2 causes a cytokine storm remains to be further explored. In addition to the above factors, there were many treatments related body reactions such as dermatitis and thyroiditis. Therefore, more effective biomarkers will be needed to block PD-1/PD-L1 in the future.
In summary, the main treatment for stage IIA NSCLC is surgery with a 5-year survival rate of about 70%. Preoperative neoadjuvant therapy is expected to improve the cure rate of surgery and prolong the survival time by reducing tumor burden. In neoadjuvant therapy, chemotherapy combined with ICIs therapy is preferred for those patients whose PD-L1 expression exceeds 50% in tumor tissues. However, ICIs treatment is not suitable for all patients. Latest studies showed that ICIs may aggravate disease progression and even cause hyper progression in patients positive for driver genes such as EGFR, ALK and ROS1, or those with mutations in genes such as mouse double minute 2 homolog (MDM2), MDM4, and serine/threonine kinase 11. In this case, the postoperative specimens showed pCR after 2 cycles of ICIs therapy combined with chemotherapy but then a cytokine storm developed. Finally, the patient died from MODS with a TTD of only 2.5 months. The retrospective genetic test of patient specimens by NGS revealed that CDKN2A and PDCD1LG2 genes were co-mutated in addition to the high expression of TMB. At present, the understanding of these genes in ICIs therapy is relatively limited with unclear functions and mechanisms.
Conclusion
According to the study reports, the mutations of CDKN2A and PDCD1LG2 are closely related to tumor progression and a variety of inflammatory diseases. Combined with the above research results, it is considered that the co-mutation of CDKN2A and PDCD1LG2 genes may cause cytokine storm in the patient, thus leading to autoimmune inflammation in multiple tissues and organs and eventually MODS. However, the specific molecular mechanism needs to be further elucidated. With the painful lessons learned from this case, it has become clear that a new strategy should comprise two trains: on the one hand, it is necessary to fully understand the patient’s physique, highrisk factors, gene expression profile with regard to neoadjuvant therapy for NSCLC, thereby avoiding serious IRAEs caused by blindly selecting ICIs, and on the other hand, it is recommended to improve the large panel genetic test with regard to molecular pathology rather than relying solely on the level of PD-L1 and then whether to complete ICIs therapy. In addition, research on the types of gene and cytokine mutations caused by ICIs should be carried out by analyzing more population subgroups.
Acknowledgements
The authors wish to thank the patient for allowing us to publish his case report and would like to express their gratitude to Edit Springs for the expert linguistic services provided.
Author Contributions
Xiguang Liu conceived the retrospective case study. Yonglong Jin identified the patient and described the clinical features; Xiguang Liu and Wenjing Xiao reviewed and revised the original. Yonglong Jin wrote the manuscript. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
Written informed consent was obtained from the patient.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The study was supported by the Natural Science Foundation of Shandong Province (ZR2019BH083).
References
- Montesion M, Murugesan K, Jin DX, Radwa S, Nora S, et al. (2021) Somatic HLA Class I Loss Is a Widespread Mechanism of Immune Evasion Which Refines the Use of Tumor Mutational Burden as a Biomarker of Checkpoint Inhibitor Response. Cancer Discov 11(2): 282-292.
- Ettinger DS, Wood DE, Aggarwal C, Dara LA, Wallace A, et al. (2019) NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 17(12): 1464-1472.
- Hussaini S, Chehade R, Boldt RG, Jacques, Phillip B, et al. (2021) Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis. Cancer Treat Rev 92:102134.
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics. CA Cancer J Clin 69(1): 7-34.
- Hui R, Özgüroğlu M, Villegas A (2019) Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol 20(12): 1670-1680.
- Forde PM, Chaft JE, Smith KN (2018) Neoadjuvant PD-1 Blockade in Resectable Lung. N Engl J Med 378(21): 1976-1986.
- Keung EZ, Ukponmwan EU, Cogdill AP, Wargo JA (2018) The Rationale and Emerging Use of Neoadjuvant Immune Checkpoint Blockade for Solid Malignancies. Ann Surg Oncol 25(7): 1814-1827.
- McGranahan N, Furness AJ, Rosenthal R, Sofie R, Rikke L, et al. (2016) Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351(6280): 1463-1469.
- Markowitz GJ, Havel LS, Crowley MJ (2018) Immune reprogramming via PD-1 inhibition enhances early-stage lung cancer survival. JCI Insight 3(13): e96836.
- Provencio-Pulla M, Nadal-Alforja E, Cobo M, Amelia I, Marinha C, et al. (2018) Neoadjuvant chemo/immunotherapy for the treatment of stages IIIA resectable non-small cell lung cancer (NSCLC): A phase II multicenter exploratory study-NADIM study-SLCG.
- Shu CA, Grigg C, Chiuzan C (2018) Neoadjuvant atezolizumab + chemotherapy in resectable non-small cell lung cancer (NSCLC)[J]. Journal of Clinical Oncology 36(15_suppl): 8532-8532.
- Tazdait M, Mezquita L, Lahmar J (2018) Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 88: 38-47.
- Cottrell TR, Thompson ED, Forde PM (2018) Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 29(8): 1853-1860.
- Oezkan F, He K, Owen D (2018) MA04.10 Comprehensive Peripheral Blood Immunophenotyping and T-Cell Clonal Analysis During Neoadjuvant Immunotherapy with Atezolizumab in NSCLC. Journal of Thoracic Oncology 13(10): S369.
- Wang DY, Salem JE, Cohen JV (2018) Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis [published correction appears in JAMA Oncol 4(12): 721-1728.
- Ishida Y, Kakiuchi N, Yoshida K (2021) Unbiased Detection of Driver Mutations in Extramammary Paget Disease. Clin Cancer Res 27(6): 1756-1765.
- Koss B, Shields BD, Taylor EM (2020) Epigenetic Control of Cdkn2a.Arf Protects Tumor-Infiltrating Lymphocytes from Metabolic Exhaustion. Cancer Res 80(21): 4707-4719.
- Garcia-Diaz A, Shin DS, Moreno BH (2017) Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression [published correction appears in Cell Rep 19(6):1 189-1201.
- Tanegashima T, Togashi Y, Azuma K (2019) Immune Suppression by PD-L2 against Spontaneous and Treatment-Related Antitumor Immunity. Clin Cancer Res 25(15): 4808-4819.






























