Mycofabrication of Silver Nanoparticles from Aspergillus Niger and Determination of its Anti-Fungal Potency Against Candida Species
Nida Tabassum Khan* and Samiullah Khan
Department of Biotechnology, Faculty of Life Sciences & Informatics, Balochistan University of Information Technology, Engineering and Management Sciences, Pakistan
Submission: April 26, 2021; Published: July 23, 2021
*Corresponding author: Nida Tabassum Khan, Department of Biotechnology, Faculty of Life Sciences & Informatics, Balochistan University of Information Technology, Engineering and Management Sciences, Balochistan, Pakistan
How to cite this article:Nida Tabassum K, Samiullah K. Mycofabrication of Silver Nanoparticles from Aspergillus Niger and Determination of its Anti- Fungal Potency Against Candida Species. Glob J Pharmaceu Sci. 2021; 8(5): 555748. DOI: 10.19080/GJPPS.2021.08.555748.
Abstract
Besides many synthetic approaches being used for the preparation of nanoparticles, bio-based synthesis of nano particles using nano factories such as microorganisms is a novel environmentally friendly approach for producing nanoparticles of definite size and shape with distinctive physical and chemical properties. This method is not only ecofriendly but provides a clean and nontoxic way for the fabrication and assembly of metallic nano sized particles. Therefore, mycosynthesis of colloidal silver nanoparticle from Aspergillus niger was achieved using fungal filtrate incubated with aqueous silver nitrate for a specific period of time as monitored by Ultraviolet-visible spectroscopy. Optimization studies of physioculture parameters such as time of incubation, pH, biomass, and substrate concentration was achieved to determine optimum conditions. It was observed that the optimum conditions were found to be 55 hours of incubation time at pH 9.0 and 6 mM of silver nitrate concentration using fungal biomass of 25g for method I and for method II were found to be after 40hours of incubation time at pH 8.0 and silver nitrate concentration of 2mM using fungal biomass of 15g. Furthermore, silver nanoparticles were found to be an effective antifungal agent against Candida species. Thus, mycofabrication of silver nanoparticles is the most reliable, inexpensive, and ecofriendly method. However, in future, identification of different eukaryotic fungal strains for the production of Silver nanoparticles is required to make this process commercially practicable.
\Keywords: Mycoynthesis; Aspergillus niger; Beer-lambert Law; Anti-fungal; Candida
Introduction
Nanotechnology is rapidly growing and has been used in the manufacturing of a wide range of commercial products on large scale. Among different types of metallic nanoparticles silver nanoparticles synthesis is gaining momentum because of its potential applications in different areas as in electronics, textiles, sensors, food preservation, paints, cosmetics [1]. Nanoparticles of silver is reported to have anti-inflammatory effects as well as antibacterial and antifungal activity. Moreover, it can be employed in anti-septic coats and dressings [1,2].
The biological synthesis of nanomaterials is a leading approach as it combines nanotechnology with biotechnology [3]. Biological organisms, such as bacteria, yeasts and filamentous fungi is known to involve in bioremediation of poisonous metal ions by reduction; hence, these organisms could be used as nanofactories for nanoparticle fabrication. Several studies reported that metallic nanoparticles of different elements such as titanium, gold, silver, platinum, palladium, selenium, tellurium, silica, magnetite and zirconium could be produced using actinomycetes, bacteria, viruses and fungi [3]. As compared to bacteria, fungi were reported to secrete higher quantities of bio-active compounds, which made fungi more suitable for commercial scale production [3]. In addition, extracellular synthesis using fungi makes downstream processing much simple and easier. An interesting example of mycosynthesis using fungi was the cell-mediated production of silver using Aspergillus niger and the particles were quasi-spherical in shape with size range between 5-15nm [4]. There are numerous reports on the synthesis of AgNPs from Fusarium acuminatum [5]. and Penicillium fellutanum [6].
Despite these findings the production of nanoparticles using fungi is still limited and in the initial phase more over their mechanism is also not completely documented. Previous research studies have reported that high concentration of active proteins secreted by fungi is involved in the reduction and capping of metal ions during nanoparticle synthesis reaction [7]. Therefore, it is ofgreat importance to discover new fungal strains for producing nanoparticles based on their diversity and understanding their molecular mechanism of production.
Aspergillus species are widely distributed in soil, water, subterranean and aerial plant parts, plant debris and other organic substrates because of their efficient dispersal mechanisms and ability to grow on different substrates [8]. Most of Aspergillus species are harmless saprobes and members of the normal soil microbial communities; however, many species are mycotoxin producers and are plants and human’s pathogen. This research aimed to study the extracellular AgNPs fabrication using an environmentally friendly approach by utilizing Aspergillus niger under laboratory conditions. Various features of AgNPs will be determined using standard techniques. Efforts will also be focused to investigate their antimicrobial activity against pathogenic microbes.
Materials And Methods
Production of Aspergillus niger biomass
Preparation of the culture medium
CD (Cezapex Dox) liquid broth was prepared by dissolving glucose (10g), yeast extract(1g), ferrous sulphate (0.01g), zinc sulphate (0.01g), calcium chloride (0.5g), magnesium sulphate (0.5g), potassium dihydrogen phosphate(1g) and sodium nitrate (2g) in 1000ml distilled H2O.
Inoculation of the culture medium
Identified Aspergillus niger strains available at Yeast & Fungal Biotechnology Lab, BUITEMS was used. The media was inoculated in sterile air under Laminar flow cabinet by using sterilized wire loop method Inoculated flasks were then placed at 150rpm for 120 hours on a shaker at r.t.p.
Fungal LCF (Live cell filtrate)
Harvesting of the fungal mycelia after 120 by Whatman’s filter paper no.1 (Figure 1). Removal of media components from the mycelia and filtrate was done obtained by washing with distilled H2O.Obtained filtrate was further filtered using PVDF (polyvinylidene fluoride) membrane having a pore size of 0.22μm with diameter of 25mm attached to a syringe to obtain cell free filtrate by removing any remaining cell debris (Figure 2). Filtrate was then centrifuged for 20min at 15000rpm. The harvested biomass was saved for later use.


Synthesis of AgNPs
Method I
20ml of centrifuged filtrate (supernatant) was brought in contact with 150ml of silver nitrate solution (1mM) [9]. The flask was covered with aluminum foil to avoid photochemical reaction and kept for 2 days in an incubator set at a temperature of 25°C. Freshly prepared CD broth incubated with aqueous silver nitrate was used as a control.
Method II
Typically, 20g of harvested mycelia (wet weight) was incubated in 200ml distilled water for 72hours at room temperature (Figure 3) and agitated at 140rpm on a rotatory shaker. Cell free filtrate was then centrifuged. 20ml of supernatant was treated with 150ml aqueous silver nitrate and incubated for two days. Distill water with aqueous silver nitrate was the control.

Confirmation of silver nanoparticle formation
Silver nanoparticle formation was visually seen by the color change in experimental flask. UV -Visible Spectroscopy (JENWAY 6305) was carried out on 1 ml of the sample in quartz micro cuvette withdrawn after 48 h for both the methods I and II from the experimental flasks to confirm and monitor the formation of AgNPs [10] (Figure 4). Absorbance peak were obtained from 360-460nm for both the methods I and II to determine optimum wavelength. O.D of distill H2O was used as a control.

Calculation of silver nanoparticles concentration at optimum wavelength (λmax) for each of the optimized parameters for method I and II was achieved by using Beer-Lambert Law [11,12]. Beer-Lambert Law: A=ℇ. L. C
Where A= absorbance (AU unit)
L= path length (Cm)
C = concentration (mol/L)
ℇ=molar extinction coefficient (M-1Cm-1)
Optimization Studies
Optimization Studies of the reaction factors such as incubation time, silver nitrate concentration, fungal mycelia and pH was done by optimizing one parameter at a time using JENWAY 6305 UV/Vis spectrophotometer required for silver nanoparticles mycosynthesis. O.D of distill H2O was used as a control.
Effect of incubation time
Optimization studies with respect to this parameter was carried out for both the methods I and II using 10ml of fungal live cell filtrate (LCF) incubated with 1mM silver nitrate which was placed in dark at 25°C in an incubator at five different incubation hours i.e., 10h, 25hrs, 40hrs, 55hrs and 70hrs with difference of 15 hrs. Using UV/Vis spectrophotometer to determine the optimum incubation period for the assembly of nanoparticles of silver.
Effect of pH
For both the methods I and II, five test tubes, each containing 10ml of fungal live cell filtrate (LCF) having five different pH i.e, 5, 6, 7, 8 and 9 was incubated with 1mM silver nitrate solution at 25°C in the dark for 48 hours. UV-visible spectroscopy was done to determine optimum pH value. pH of the fungal filtrate was adjusted using1N Hydrochloric acid and 1N Sodium hydroxide solutions.
Effect of AgNO3 Concentration
In this case for both the methods I and II, five test tubes each containing 10ml of fungal live cell filtrate (LCF) was incubated with different concentration of AgNO3 i.e., 2mM, 4mM, 6mM, 8mM and 10mM with difference of 1mM incubated at 25°C in the dark for 48 hours. UV-visible spectroscopy was done to determine optimum silver nitrate concentration for the production of nano silver particles.
Effect of fungal biomass concentration
Five different fungal wet biomasses i.e., 5g, 10g, 15g, 20g and 25g was used to study the effect of different biomass concentration on silver nanoparticles synthesis. For method I different grams of wet biomass was kept in five conical flasks each containing 250ml of freshly prepared CD liquid broth and for method II the different grams of the wet biomass of the fungus was kept in in five conical flasks each containing 250ml of distill water for 72hrs i.e. 3 days on a rotatory shaker at 140rpm at r.t.p. 10ml of the obtained filtrate from each flask was than incubated with 1mM silver nitrate at 25°C for 48 hrs. UV-visible absorption spectroscopy was used to find out optimum fungal biomass.
Determination of antimicrobial activity of silver nanoparticles
Agar disc diffusion method was used to evaluate antimicrobial effectiveness of the mycosynthesized silver nanoparticles against various pathogens [13].
Preparation of the YM agar plates
YM agar prepared by dissolving Agar (20g), Glucose (10g), Malt Extract (3g), Peptone (5g), and Yeast Extract (3g) in 1000ml of distilled H2O and was autoclaved. 1ml of 10% sterilized tartaric acid and 0.5ml antibiotic ampicillin was added to adjust medium pH (4.2-4.5) and inhibit bacterial contamination, respectively. Agar surface was inoculated with Candida glabrata, Candida albicans and Candida tropicalis.
Preparation of sterile discs
1cm sized sterilized discs were impregnated with different concentration of silver nanoparticle solution (20 μL, 40 μL, 60 μL and 80 μL). (Figure 5) and placed on agar surface, incubated for 48 hrs at 37 °C. Diameter of the inhibition zone was measured using a caliper. Aqueous silver nitrate impregnated discs were the controls.

Results
In current study mycofabrication, optimization of biosynthesis parameters of silver nanoparticles and determination of its antimicrobial activity was successfully accomplished.
Aspergillus Niger biomass production
After incubation for 120 hours sufficient biomass of Aspergillus Niger was obtained Figure 6.

Biosynthesis of silver nanoparticles
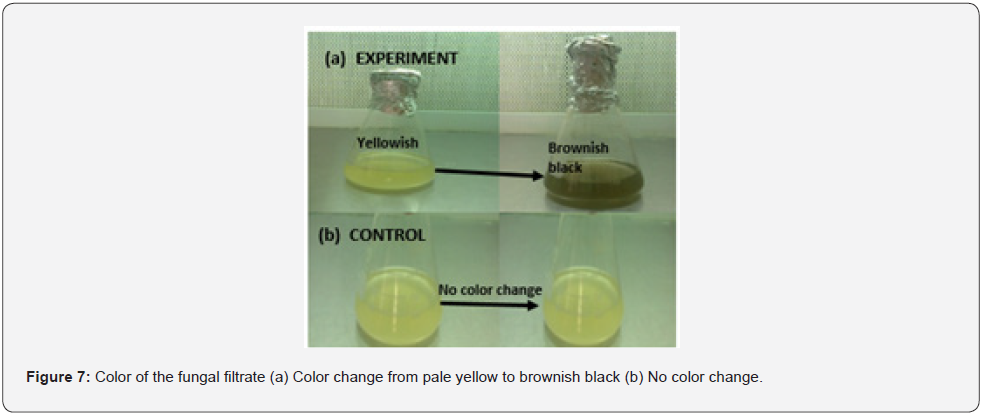
For method I and method II, gradual change in the color was seen from pale yellow to brownish black after 48 hours of incubation of the experimental flasks with silver nitrate. However, no color change was observed in the controls Figure 7.
Ultraviolet Visible Spectroscopy
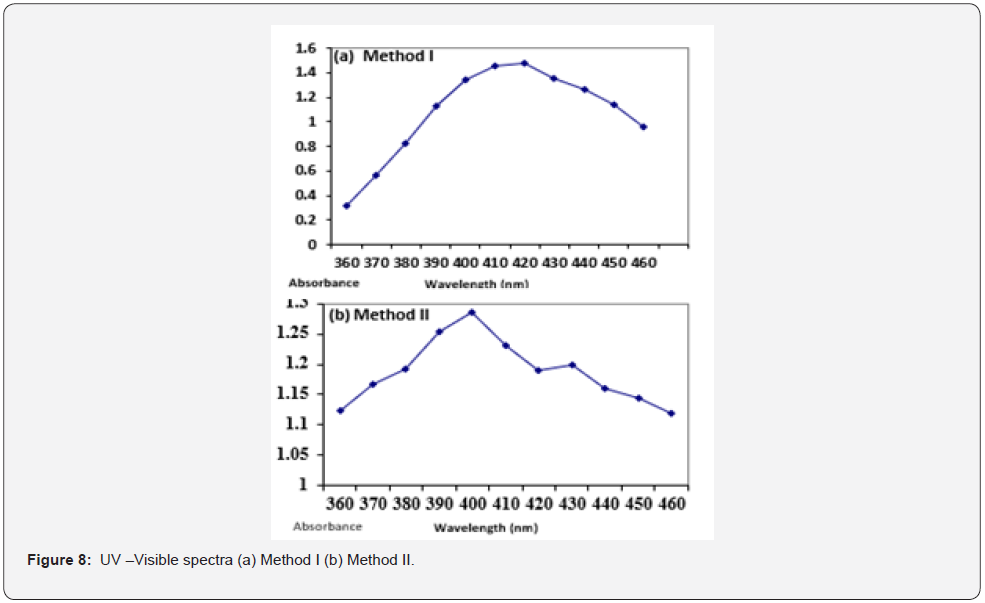
Visible Spectroscopy results showed that for method I and II a peak at 420nm and 400nm was seen respectively (Figure 8).
Optimization of parameters
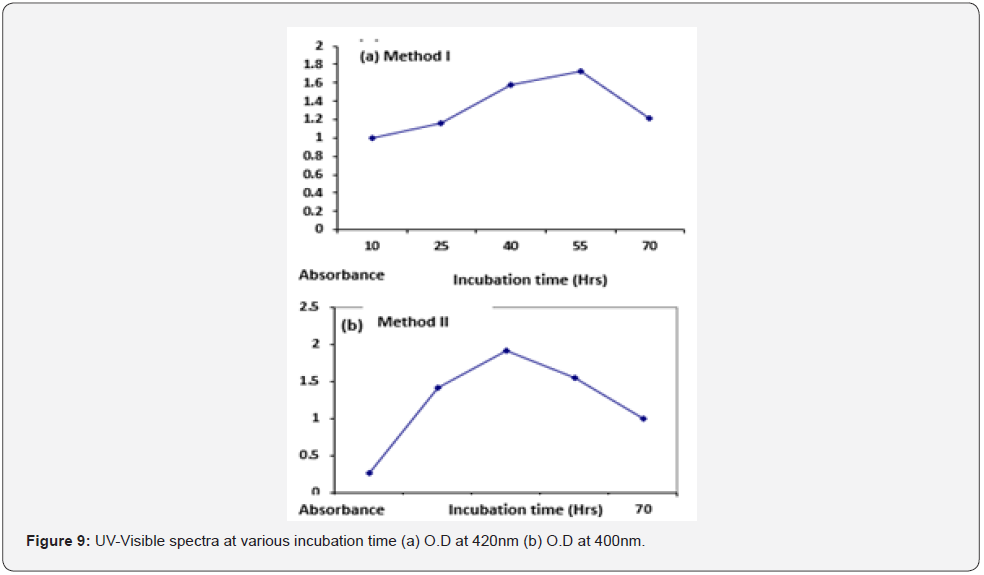
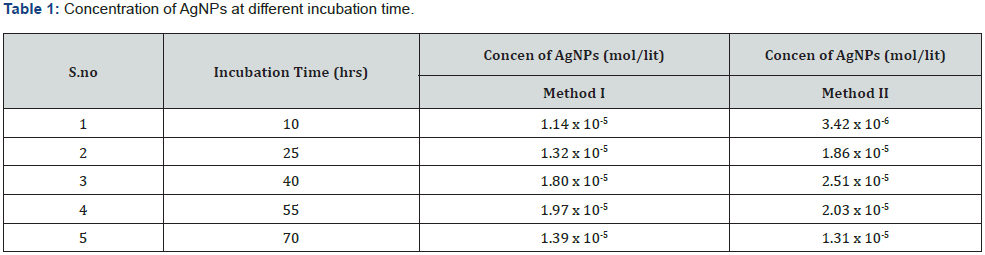
Effect of incubation time: For method I maximum absorbance was seen at 55hrs of incubation time (O.D =1.73) while for method II it was obtained at 40hrs of incubation (O.D =1.91). O.D of distill H2O was measured to be zero. Concentration of AgNPs at different incubation time for both the methods is given in (Table 1). UVVisible spectra at different incubation time for both the methods I and II is given below Figure 9.
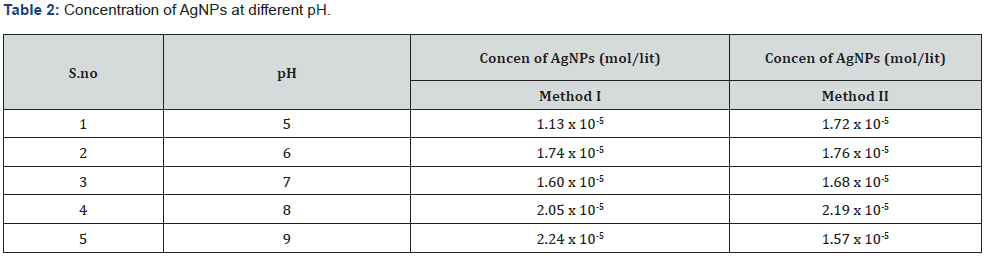
Effect of pH: For method I and II the highest absorbance value was obtained at pH 9 (O.D =1.97) and pH 8 (O.D = 1.67) respectively. Optical density of distill water was measured to be zero. Concentration of AgNPs at different pH for both the methods is given in (Table 2). UV-Visible spectrum of silver nanoparticles produced at various pH for both the methods is given below Figure 10.
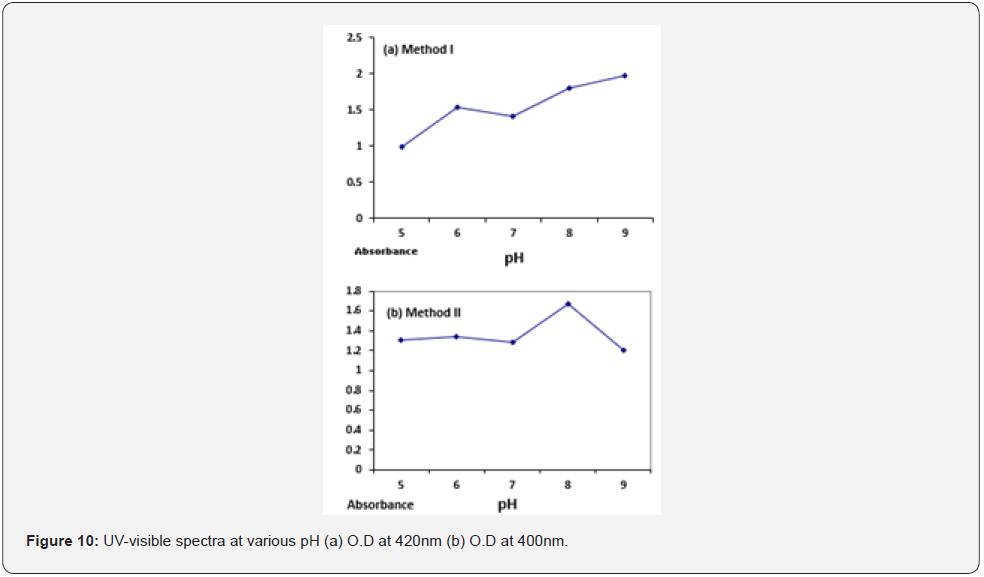
Effect of AGNO3 concentration: UV-visible absorption spectra revealed that highest absorption value was obtained at 6mM AgNO3 (O. D= 1.71) for method I and for method II maximum absorbance value was at 2mM of AgNO3 (O. D= 1.89). Concentration of AgNPs at different pH for both the methods is given in (Table 3). UV-Visible spectrum of silver nanoparticles synthesized at various concentration of AgNO3 is given below Figure 11.
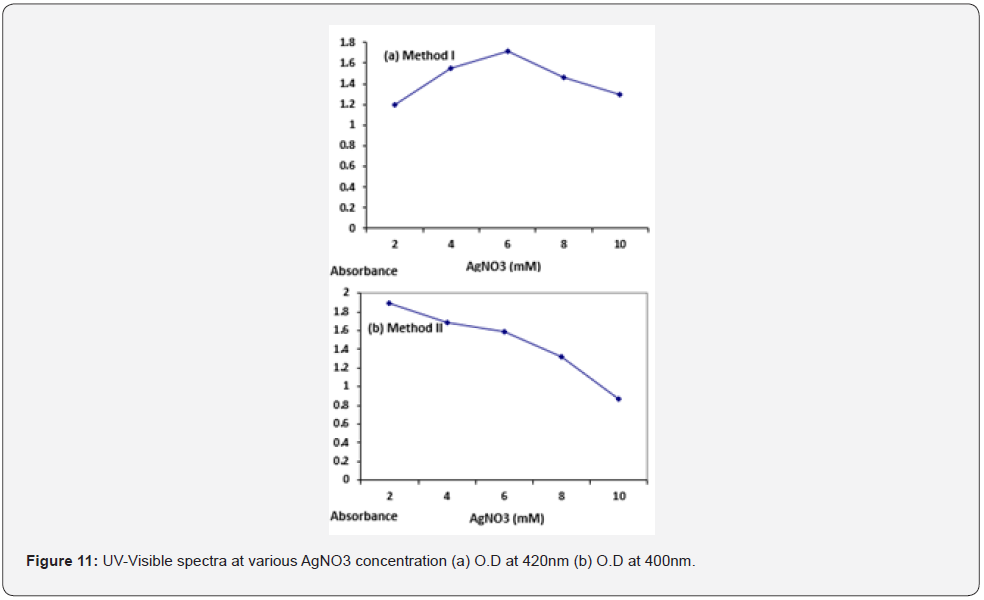
Effect of biomass concentration: Highest absorbance peak was obtaining at 25g of fungal biomass (O. D= 1.95) for method I while for method II highest peak was obtained at 15g of fungal biomass (O. D= 1.48) (Figure 12). Optical density of distill water was measured to be zero. Concentration of AgNPs at different pH for both the methods is given in Table 4.
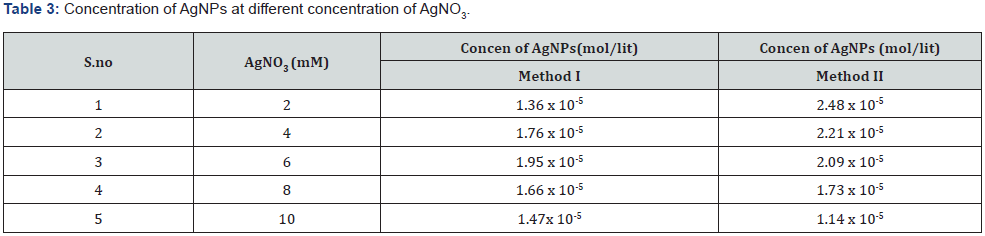
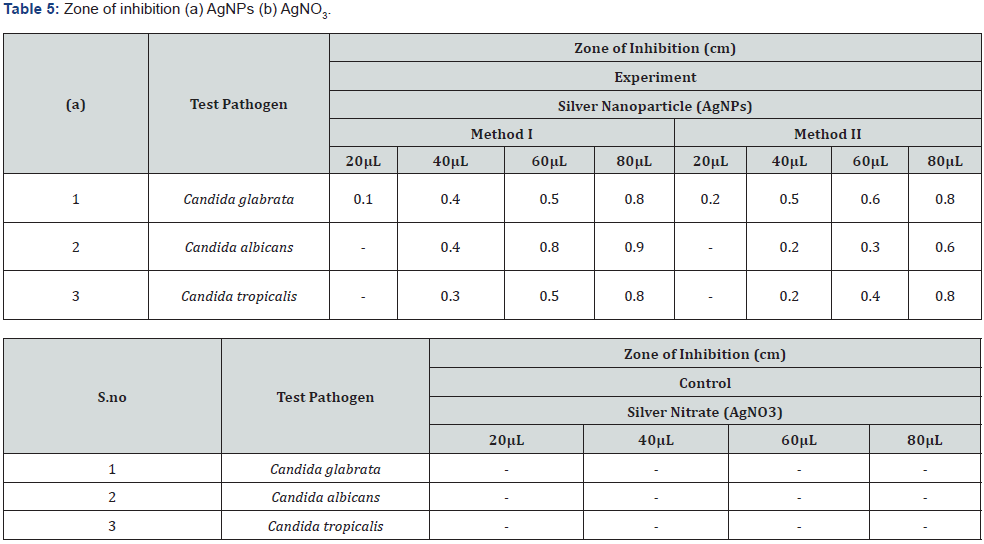
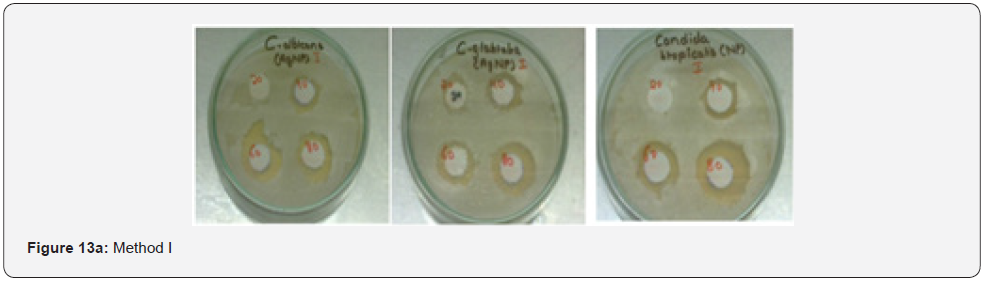
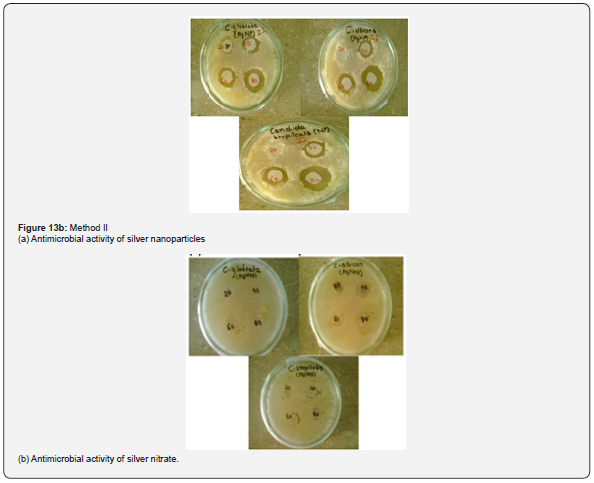
Antimicrobial activity of AgNPs: The colloidal suspension of mycosynthesized silver nanoparticles obtained from Method I and II was found to possess antimicrobial potency against candida species as indicated by a clear zone around silver nanoparticles impregnated discs (Figure 13). However, no zone of inhibition was seen around silver nitrate impregnated discs Table 5.

Discussion
Silver nanoparticles were mycofabricated using Aspergillus niger, an environmentally friendly green approach [14]. As the fungus was given sufficient time to use the available nutrients in the broth therefore maximum biomass was obtained. Fungal mycelia were harvested and incubated in distill water to obtain filtrate which could be used for silver nanoparticle fabrication. Thus, biosynthesis of silver nano crystals in in vitro was achieved by using these two approaches. Experimental flask for method I containing fungal filtrate turned brownish black upon silver nitrate addition indicating reduction of silver ions to silver nanoparticles [15-18]. However, in contrast no change in color was observed in the controls. For method II, after the addition of silver nitrate solution the color changes from colorless to light brown/ black. This color change is due to the presence of nitrate reductase in the fungal filtrate which causes reduction of silver ions to form the corresponding nanoparticles [19-20]. Absence of this enzyme in the control flasks indicates that silver ions has not been reduced therefore no change in color was seen.
Due to excitation of surface plasmon resonance (SPR) in the metal nanoparticles, change in color was observed [21-23]. In the present research SPR occurred in the visible region with absorbance at 420 nm for method I and 400nm for method II. Silver nanoparticles were well dispersed in the solution with not much aggregation and remained stable for weeks [20]. Since external conditions influence the metabolic activity of an organism. Therefore, protein expression is affected by the external environments in which the fungi is cultured. Present optimization studies for method I and II, the optimum time of incubation was found to be 55hrs and 40hrs, respectively. The rate of AgNPs synthesis is directly proportional to the incubation time of the fungal filtrate with silver nitrate solution. The obtained results clearly revealed that the rate of silver ions reduction and AgNPs formation was going slowly at 10 and 25hours of incubation for both the methods I and II, as indicated by the low concentration of silver nanoparticles (Table 1). But with increase in reaction time the peak becomes sharper. As the time required for the completion of reaction increases, more silver nanoparticles were formed therefore maximum silver ions were reduced after 48hrs for method I and at 40hrs for method II. The increase in absorbance along with color intensity could be backed to an increase the amount of AgNPs formed with time [24,25]. However, an optimum duration is required for silver nanoparticles synthesis, as silver nanoparticles agglomeration occurs after the optimum duration resulting in larger particle sizes [26] as indicated by the spectrum.
Optimum pH value for method I and method II were found to be 9 and 8 respectively as indicated by the absorbance value at pH 9 (O.D =1.92) and pH 8 (O.D = 1.67) measured at 420nm and 400 nm respectively. Absorbance peak at pH 9 indicates maximum synthesis of silver nanoparticles i.e., 2.24 x 10-5 mol/l because of the availability of numerous chemically reactive groups for silver ion binding at alkaline pH thus assisting reduction reaction [27]. On the contrary at low pH values, protein activity is affected thus rate of nanoparticles synthesis decreases i.e., 1.13 x 10-5 mol/l [28]. In case of method II optimum pH was found to be 8 at a wavelength of 400 which indicates the stability of enzyme nitrate reductase. However, increase in pH affects the activity of the enzyme thus reduces the rate of silver ions reduction causing a decrease in the number of silver nanoparticles i.e., 1.57 x 10-5 mol/l and aggregates were observed as indicated by the decline in the UV spectrum. Therefore, it is concluded that nitrate reductase secreted by Aspergillus Niger in the aqueous filtrate is stable at alkaline pH 9 and pH 8 for method I and method II, respectively. Silver nanoparticles were shown to be monodispersed but not at strong alkaline or acidic pH values where silver nanoparticles aggregations were observed. However, at neutral pH there was little synthesis of silver nanoparticles for both the methods since no peak was observed as indicated by the UV spectrum. Optimal AgNO3 concentration was determined to be 6 mM and 2 mM for methods I and II at a specific wavelength of 420nm and 400nm respectively. When silver nitrate concentration was increased to 6mM, so the concentration of silver ions for bio reduction also increases. Beyond this concentration there is no further increase in the rate of synthesis of silver nanoparticles (Figure 12) [29]. Absorbance and sharpness of peak increases at higher silver ion concentration showing increase in silver nanoparticle size but no significant absorption peak was found at higher silver nitrate concentrations. For method II 2mM of AgNO3 was found to be optimum beyond this increasing silver nitrate concentration means that the amount of substrate exceeds the amount of available nitrate reductase required for silver nanoparticle synthesis and this amount of silver nitrate has toxic effects on the enzyme therefore reduction rate of silver ions is extremely decreased as indicated by a sharp decline in the obtained UV spectra (Figure 11).
Mycelia plays a central role in silver ion reduction. High concentration of fungal mycelia means higher concentration of protein in broth consequently silver ions will be reduced at a faster rate as indicated by the spectrum (Figure 12) obtained for method I that maximum was observed at 25g of fungal mycelia. Because of the utilization of the sugar glucose by the fungus Aspergillus niger present in the media resulted in increased metabolic activity of the fungus as observed by means of rapid growth of the fungal mycelia thus releasing increased concentration of the protein at a faster rate .However for method II 15g of wet biomass was found to be optimal for silver nanoparticle formation because since the fungal biomass was incubated in distill water which lacks the carbon source therefore no growth of the fungal mycelia was observed. Hence the obtained live cell filtrate contains moderate concentration of the enzyme required for silver nanoparticles formation as indicated by the UV. vis spectra (Figure 12) that increasing the concentration of fungal biomass beyond 15g lowers the synthesis.
Metallic silver in the form of nanoparticles shows antimicrobial activity against many microorganisms [30]. The obtained results suggested that AgNPs produced by Aspergillus Niger showed potent antifungal activity against Candida species as indicated by clear zone around silver nanoparticle impregnated discs. The possible reason behind fungal growth inhibition is by means of sulfhydryl group inactivation in the fungal cell wall due to formation of insoluble compounds which disrupts membrane bound lipids and proteins resulting in cell lysis [20]. Similar results were reported by [31] using silver nanoparticles synthesized at different physiochemical conditions. Concentration of silver nanoparticles is responsible for its antimicrobial spectrum as shown by a wider zone of inhibition when increased concentration of the colloidal silver nanoparticles were used against these pathogenic fungal strains .However a low concentration of 20μL has no effect on Candida species as indicated by absence of inhibition zone and a very narrow spectrum was seen against Candida glabrata but as the concentration was increased from 20μL to 40μL then 60μL and 80μL the measured diameter of the inhibition zone was found to be wider showing broad antimicrobial spectrum of the silver nanoparticles against these fungal strains [32] (Figure 13).
Conclusion
Exploration of biological systems for the amalgamation of different metallic nanoparticles is gaining momentum now a days. By utilizing metal ion reduction capabilities of biological organisms, extracellular synthesis of silver nanoparticles was achieved by using a fungi. By monitoring environmental parameters, stable silver nanoparticles of different morphologies could be produced with effective antimicrobial potential that could be considered as a potential antifungal agent implicating its biomedical application.
References
- Ahamed M, AlSalhi MS, Siddiqui MKJ (2010) Silver nanoparticle applications and human health. Clin Chim Acta 411(23-24): 1841-1848.
- Chaloupka K, Malam Y, Seifalian AM (2010) Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol 28(11): 580-588.
- Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156(1-2): 1-13.
- Ahmad A, Senapati S, Khan MI, Kumar R, Ramani R, et al. (2003) Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete. Rhodococcus species Nanotechnology 14: 824-828.
- Ingle A, Gade A, Pierrat S, So¨nnichsen C, Rai M (2008) Mycosynthesis of silver nanoparticles using the fungus acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci 4(2): 141-144.
- Kathiresan K, Manivannan S, Nabeel AM, Dhivya B (2009) Studies on silver nanoparticles synthesized by a marine fungus Penicillum fellutanum isolated from coastal mangrove sediment. Colloids Surf B 71(1): 133–137.
- Bharde A, Rautray D, Bansal V, Ahmad A, Sarkar I, et al. (2006) Extracellular Biosynthesis of Magnetite using fungi. Small 2(1): 135-141.
- Bansal V, Rautray D, Bharde A, Ahire K, Sanyal A, et al. (2005) Fungus-mediated biosynthesis of silica and Titania particles. Journal of Materials Chemistry 15: 2583-2589.
- Sastry M, Ahmad A, Khan MI, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and Actinomycetes. Curr Sci 85: 162-170.
- Sun YP, Atorngitjawat P and Meziani MJ (2001) Preparation of Silver Nanoparticles via Rapid Expansion of Water in Carbon Dioxide Microemulsion into Reductant Solution. Langmuir 17: 5707-5710.
- Euser TG, Chen TSY (2008) Appl Phys 103 (10): 103108.
- Maxwell DJ, Taylor Ernie S (2002) J Am Chem Soc 124: 9606.
- Kiehlbauch J A, Hannet G E, Salfinger M, Archinal W, Monserrat C, et al. (2000) Use of National Committee for Clinical laboratory Standards guidelines for disk diffusion susceptibility testing in New York state laboratories. J Clin Microbial 38: 3341-48.
- Qian Y, Yu H, He D, Yang H, Wang W, et al. (2013) Biosynthesis of silver nanoparticles by the endophytic fungus Epicoccum nigrum and their activity against pathogenic fungi. Bioprocess and Biosystems Engineering 36(11): 1613-1619.
- Jae YS and Beom SK (2009) Rapid Biological Synthesis of Silver Nanoparticles Using Plant Leaf Extracts. Bioprocess Biosyst Eng 32: 79-84.
- (1991) There is plenty of room at the bottom. Science 2549: 1300-1301.
- Balaji DS, Basavaraja S, Deshpande R, Bedre D, Prabhakar BK, et al. (2009) Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids and Surfaces B Biointerfaces 68(1): 88-92.
- Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, et al. (2001) Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano 1: 515-519.
- Anil Kumar S, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, et al. (2007) Biotechnol Lett 29,439.
- Durán N, Marcato PD, Alves OL, de Souza GIH, Esposito E (2005) Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum J Nanobiotechnology 3: 1-8.
- Henglein A (1993) Physicochemical Properties of Small Metal Particles in Solution: ‘Microelectrode’ Reactions, Chemisorption, Composite Metal Particles, and the Atom-to- Metal Transition. Phys Chem B 97: 5457-5471.
- Sastry M, Mayya KS, Bandyopadhyay K (1997) pH Dependent Changes in the Optical Properties of Carboxylic Acid Derivatized Silver Colloidal Particles. Colloids Surf A 127: 221-228.
- Sastry M, Patil V, Sainkar SR (1998) Electrostatically Controlled Diffusion of Carboxylic Acid Derivatized Silver Colloidal Particles in Thermally Evaporated Fatty Amine Films Phys Chem B 102: 1404-1410.
- Park T (2007) Nano letters 7(3): 766-772.
- Bhainsa KC, D Souza SF (2006) Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids and Surfaces B: Biointerfaces 47: 160-164.
- Dubey SP, Lahtinen M, Sarkka H, Sillanpaa M (2010) Bioprospective of Sorbus aucuparia leaf extract in development of silver and goldnanocolloids. Colloids and Surfaces B: Journal of Biointerfaces 80: 26-33.
- Sathishkumar M, Sneha K, Yun YS (2010) Immobilization of silver nanoparticles.
- Banu A, Rathod V, and Ranganath E (2011) Silver nanoparticle production by Rhizopus stolonifer and its antibacterial activity against extended spectrum 𝛽-lactamase producing (ESBL) strains of Enterobacteriaceae. Materials Research Bulletin 46(9): 1417-1423.
- Rao CRK, Trivedi DC (2005) Synthesis and characterization of fatty acids passivated silver nanoparticles—their interaction with PPy. Synthetic Metals 155(2): 324-327.
- Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances 27(1): 76-83.
- Nayak RR, Pradhan N, Behera D et al. (2011) Green synthesis of silver nanoparticle by Penicillium purpurogenum NPMF: the process and optimization. Journal of Nanoparticle Research 13(8): 3129-3137.
- Synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Journal of Bioresource Technology 101: 7958-7965.






























