In vitro assessment of the synergism between extracts of Zanthoxylum zanthoxyloides and Zanthoxylum leprieurii and some standard antibiotics
Marta Gonçalves1,2 Ana M Madureira3, Luís Catarino4, Ana Monteiro1 and Generosa Teixeira4,5*
1LEAF, Instituto Superior de Agronomia, Universidade de Lisboa, 1349-017, Lisboa, Portugal.
2Adv. Inst. Nanotechnology (SAINT), Sungkyunkwan University, Suwon 16419, South Korea.
3iMed, Faculdade de Farmácia, Universidade de Lisboa, 1649-003, Lisboa, Portugal.
4cE3c, Faculdade de Ciências, Universidade de Lisboa, 1749-016 Lisboa, Portugal.
5DCFM, Faculdade de Farmácia, Universidade de Lisboa, 1649-003, Lisboa, Portugal.
Submission: May 25, 2021; Published: June 22, 2021
*Corresponding author: Generosa Teixeira
How to cite this article:Marta G, Ana M, Madureira, Luís C, Ana M, Generosa T. In vitro Assessment of the Synergism between Extracts of Zanthoxylum Zanthoxyloides And Zanthoxylum Ieprieurii and Some Standard Antibiotics . Glob J Pharmaceu Sci. 2021; 8(3): 555742. DOI: 10.19080/GJPPS.2021.08.555742.
Abstract
Purpose: To survive in harsh environments, plants developed functional and metabolic adaptive mechanisms. One of the most relevant defense strategies is the biosynthesis of secondary metabolites, including terpenoids, alkaloids, flavonoids, and phenolics that are accumulated in cellular organelles or secretory structures. Hence, plants are recognized as a valuable source of natural products and for thousands of years very diverse herbal formulations were created to treat several diseases. Zanthoxylum zanthoxyloides and Zanthoxylum leprieurii, two Rutaceae species native to Guinea-Bissau, are well known for their ethnopharmacological relevance.
Methods: In the present study, the in vitro antimicrobial activity of these plants against human pathogens was assessed and the phytochemical profile was screened. The extracts of roots and young leaves were obtained by sequential extraction of increasing polarity (n-hexane, CH2Cl2, EtOAc, MeOH and H2O) and tested against Gram-positive and Gram-negative bacteria. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined, as well as the evaluation of the synergic potential of the extracts.
Results: Z. leprieurii leaves extracts, the most apolar ones, had the highest antimicrobial activity, being able to inhibit the growth of Enterococcus hirae and all the Staphylococcus strains assayed, including the resistant ones. A synergic effect between the Zanthoxylum species extracts and standard antibiotics was found, reverting the activity of resistant strains. The phytochemical screening revealed the presence of terpenes, flavonoids, and phenolic compounds, known to have antibacterial properties.
Conclusions: The obtained results point to the validation of their use in tradition medicine and emphasize the worthwhile of additional studies of these species to better understand the compounds and mechanisms that may be valuable to restore antibacterial activity.
Keywords: Zanthoxylum zanthoxyloides; Zanthoxylum leprieurii; Antimicrobial activity; antibiotics; Synergic effect; Bioactivity
Abbreviations: MIC: Minimum Inhibitory Concentration; MBC: Minimum Bactericidal Concentration; LISC: Lisbon University Herbarium
Introduction
The use of plants as a medicine has been reported since the beginning of civilization. Diverse plant parts and extracts led to innumerous formulations created to treat a wide variety of diseases [1-3]. Nowadays, plants are still part of traditional medicine but also play a greater role on the source of new compounds that can be active by themselves or be used as lead molecules to developed new drugs.
The discovery of penicillin in the early 1940s, led to the development of several classes of compounds with antibacterial activity which allowed the reduction of mortality and morbidity caused by infectious diseases. However, the irrational and uncontrolled use of antibiotics by health professionals, animal industry and agriculture resulted in the emergence of resistant microbial populations. As a result of this strong selective pressure and increased volume of intercontinental travel, the development and transmission of multiresistant bacteria is turning into a serious public health problem [4,5].
According to [6], the mechanisms of antibiotic resistance can be summarized in four major classes: I. Modifications of the antibiotic molecule; II. Decreased antibiotic penetration and efflux; III. Changes in target sites; IV. Resistance due to global cell adaptations. The combined effects of these mechanisms associated with rapid growth rates and the ability to exchange genes led to the development of methicillin resistant (MRSA), Vancomycin Intermediate Staphylococcus aureus (VISA) and Vancomycin Resistant Enterococci (VRE). For instance, in 15 European countries more than 10% Staphylococcus aureus infections are caused by methicillin-resistant strains (MRSA), with several countries, namely Portugal presenting resistance rates closer to 50%. Several highly resistant gram-negative pathogens like Acinetobacter species, multidrug-resistant, Pseudomonas aeruginosa, carbapenem-resistant Klebsiella species and Escherichia coli are also emerging as significant pathogens, transforming antibiotic resistance in one of the greatest public health threats of the 21st century [7].
The occurrence of various infectious diseases and the increasing prevalence of antibiotic-resistant pathogens have become a serious threat to human health, and it is necessary to find new antimicrobial agents capable of reversing antibiotic resistance. Pharmaceutical companies, in the past few decades, have shifted their development efforts to chronic diseases and antiviral compounds instead of the development of new antibiotics molecules [8]. Several previous studies proved that there is a huge potential of plant-derived compounds as antibacterial and as a resistance-modifying of other antibiotics through synergistic behavior [9]. Therefore, in vitro antibacterial assessment of plants extracts, their phytochemical profile screening and the evaluation of the synergic affect when combined with antibiotics reversing the bacteria resistance may lead to a new approach on antiinfective therapy [10].
The selected species for the present study are autochthonous plants from Guinea-Bissau, a small African Portuguese speaking country, where there is a wide range of plants used in herbal medicine to treat endemic diseases as result of fragile health services, easily found in local markets and pharmacies [11]. This country is estimated to have a vascular flora with around 1507 species, 1495 of which are native. Since 1997, to protect biodiversity, a network of protected areas was established in Guinea-Bissau, a collaboration between National Institute for Biodiversity and Protected Areas and International Union for the Conservation of Nature [12]. Traditionally used and present in Guinea-Bissau flora, there are two species of Zanthoxylum with medical properties. Zanthoxylum zanthoxyloides is used to treat malaria, sick cell anemia, tuberculosis, ulcers, hemorrhoids, injuries, and syphilitic wounds as well as arthritic pain [13]. Zanthoxylum leprieurii is traditionally used in the treatment of HIV,malaria, urinary infections, rheumatic pain and used as antiseptic [14]. The present work aims to evaluate the in vitro antimicrobial activity of the two Zanthoxylum species, Z. zanthoxyloides and Z. leprieurii, against a selected panel of microorganisms, to better understand their application in traditional medicine. The potential synergistic effect of plant extracts with standard antibiotics was tested as well as screening of the phytochemical profile.
Materials and Methods
Plant materials
Samples of Z. zanthoxyloides and Z. leprieurii roots and young stems were collected during 2016-2017, in Orango island, Bagos archipelago, Guinea-Bissau and dried at room temperature. Voucher specimens were deposited and identified at the Lisbon University Herbarium (LISC).
Plant extracts
The plant material was powdered, obtaining 6g of leaves and 30g of roots of Z. zanthoxyloides and 13g of leaves and 27g of roots of Z. leprieurii. Each powdered material was subjected to extractions with five ascending polarity solvents: n-hexane (n-hex), dichloromethane (CH2Cl2), ethyl acetate (AcOEt), methanol (MeOH) and water (H2O). Ensuing the usual procedures [15] each solvent extraction took about 24h, at room temperature, with occasional shaking followed by decantation, filtration, drying and storage at -20ºC, until use.
Bacterial strains
To assess the in vitro antimicrobial activity of each plant, extract the model proposed by [16] was considered. The microorganisms include a range of Gram-positive and Gram-negative bacteria, all known to be the cause of several human diseases Table 1.
Antibiotics
The standard antibiotics amoxicillin and oxacillin, purchased from Sigma (Madrid, Spain).
Minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) values were determined by the microplate broth microdilution method according to CLSI (2019). Briefly, on each well of the microplate, 100 μL of the medium plus 100μL of each extract solution to be tested were added, obtaining concentrations ranging between 500-7.5μg/mL. An inoculum of each microorganism was also added (10μL; final concentration 104cfu/mL). Appropriated antibiotics were used as reference for antibacterial activities. After incubation at 37ºC for 24 h, the optical density at 630nm was measured in a Biotek ELX 808 plate spectrophotometer to assess the bacterial growth and confirmed by macroscopic evaluation. The samples with MIC value ≤ 100μg/mL were determined to have antibacterial activity. All assays were performed in triplicate.
Minimum bactericidal concentration (MBC)
The minimum bactericidal concentration (MBC) was evaluated following the CLSI method [17] with some modifications. 10μL of suspension from each well showing no visible growth was spread out on a Muller-Hinton agar plate. Colony growth was observed after 24h incubation at 37ºC. MBC corresponds to the lowest concentration of extract that reduces almost at 100% the viability of the bacteria.
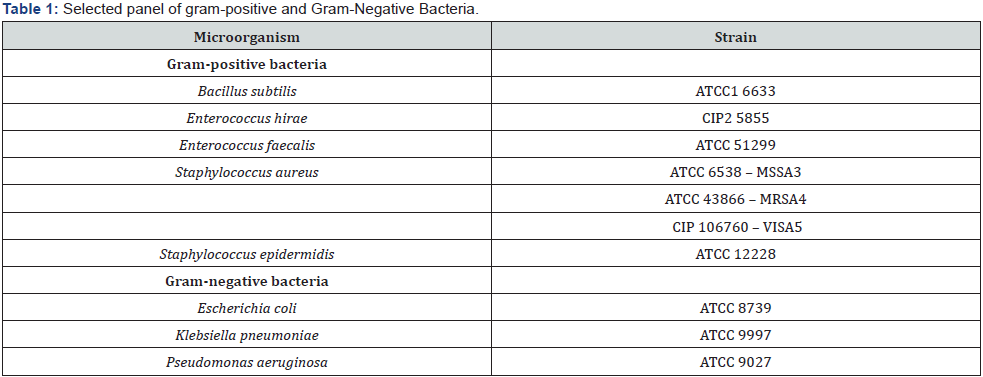
1ATCC: American Type Culture Collection, Maryland, USA; 2CIP – l’Institut Pasteur Collection, Paris, France; 3MSSA: Methicillin-Sensitive Staphylococcus aureus; 4MRSA: Methicillin-Resistant S. aureus; 5VISA: Vancomycin-Intermediate S. aureus.
Synergic effect of extracts with standard antibiotics
In order to determine the type of interaction between the extracts and the standard antibiotics (amoxicillin and oxacillin), a checkerboard assay was performed against the MRSA strain ATCC 43866 and VISA strain CIP 106760 according to [18]. Two-fold serial dilutions of antibiotic was prepared on the horizontal rows of microtiter plate and then cross- diluted vertically by two-fold serial dilutions of the extracts. The concentration of each antibiotic ranged from 1 to 1/2048 of the MIC and the concentration of the compounds from 1/2 to 1/64 of the MIC.
The synergic effect was determined based on the fractional inhibitory concentration index (FICI), calculated according to the formula
FICI= FIC(A)+FIC(B)
where, FIC(A)= MIC (A in the presence of B)/MIC (A alone) and FIC(B)= MIC (B in the presence of A)/MIC (B alone) [18]. When FICI ≤ 0.5 a synergic effect is considered. FICI ranging 0.5-4 are classified as indifference and FICI values > 4 an antagonistic effect is pondered [18].
Phytochemical screening
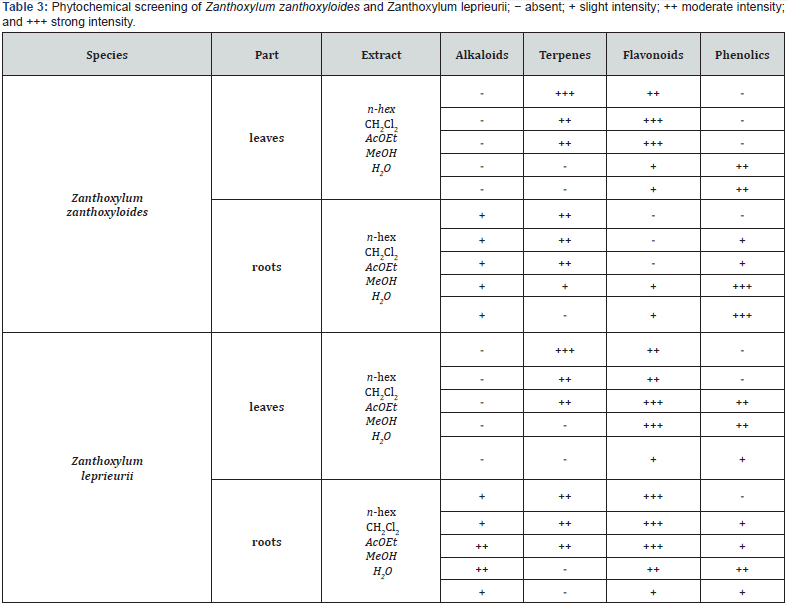
A semi-quantitative phytochemical analysis to detect the major chemical groups found in each extract was carried out through thin layer chromatography (TLC) on silica gel plates [19]. Proper mixtures of eluents were used to develop and the spots were revealed with appropriated spray-reagents made according to [20]. being: anisaldehyde-sulfuric acid reagent for terpenoids,Dragendorff reagent for alkaloids, natural products–polyethylene glycol (NEU) reagent for flavonoids and Fast Blue salt reagent for phenolic compounds. Results were displayed between absent (-) and a strong intensity (+++).
Results
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
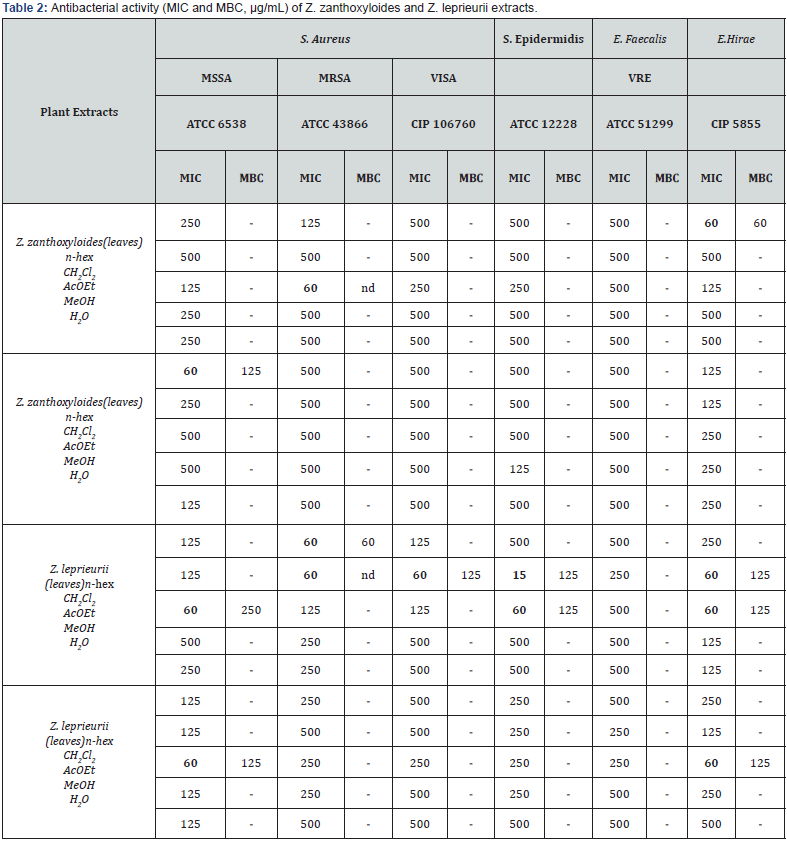
A total of 20 extracts of two Zanthoxylum species were prepared where the bactericidal and bacteriostatic activity (MBC and MIC) were evaluated against an enlarged panel of Gram positive and Gram negative sensitive and resistant bacteria strains. The results are presented in Table 2.
The MSSA growth was inhibited by Z. zanthoxyloides n-hex root extract (MIC 60µg/mL) and by both Z. leprieurii leaves and roots AcOEt extracts (MIC 60µg/mL). Z. zanthoxyloides AcOEt leaves extract and Z. leprieurii n-hex and CH2Cl2 leaves extracts inhibit the development of the MRSA strains (MIC 60 µg/mL). The VISA strain was inhibited by Z. leprieurii CH2Cl2 leaves extract (MIC 60µg/mL). Z. leprieurii CH2Cl2 and AcOEt leaves extracts also presented activity against S. epidermidis (MIC respectively 15 and 60µg/mL). Z. zanthoxyloides leaves n- hex extract, Z. leprieurii leaves CH2Cl2 and AcOEt extracts and Z. leprieurii roots AcOEt extract exhibited inhibitory activity against E. hirae (MIC 60μg/mL). The most active plant part was the Z. leprieurii leaves and the extract presenting major activity was the CH2Cl2 extract which displayed activity against MRSA, VISA, S. epidermidis and E. hirae strains.
Synergic effect of extracts with standard antibiotics
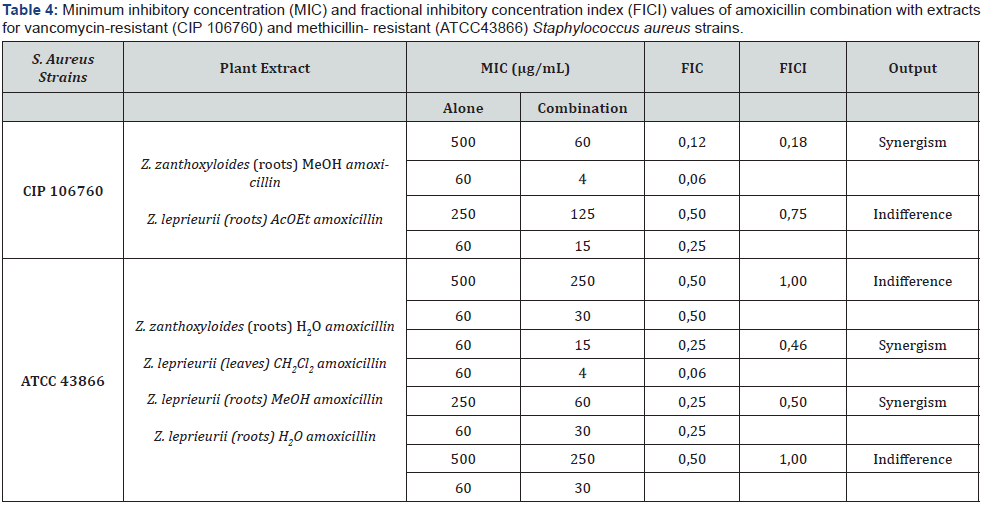
In order to evaluate the interactions between the extracts and two reference antibiotics used to treat S. aureus infections (amoxicillin and oxacillin), the checkerboard assay was performed with two S. aureus strains one methicillin-resistant (MRSA, ATCC 43866) and vancomycin-resistant (VISA, CIP 106760). The results for extracts with fractional inhibitory concentration index (FICI) 1 or lower are displayed in Table 4 and Table 5. FICI values under 0.5 are considered to show a synergic interaction between the antibiotic and the plant extracts [18].
Phytochemical screening
Results are displayed in Table 3.

MSSA: Methicillin-Sensitive Staphylococcus aureus; MRSA: Methicillin-Resistant Staphylococcus aureus; VISA: Vancomycin- Intermediate Staphylococcus aureus; VRE: Vancomycin-Resistant Enterococcus; Nd: Non-Defined; (-) not tested.
Discussion
Analyzing the results displayed in (Table 2), the bioactive extracts are the non-polar ones, mainly CH2Cl2 and AcOEt extracts which are rich in terpenes and flavonoids (Table 3). Terpenoids have antibacterial properties in nature, acting by an unclear mechanism. They seem to disrupt the membrane of bacteria cell [8]. Flavonoid’s bioactivity is markedly related with the antioxidant action. Nevertheless, several properties have been reported as anti- inflammatory, antimicrobial, antiviral, antiallergic and antitumor [21-23].
No antibacterial activity was found for Gram-negative bacteria and B. subtilis. The presence of a highly hydrophobic outer membrane that works as a permeability barrier in this group, can explain those results [24]. Comparing the MIC / MBC values (Table 2), it is possible to deduce that the extracts that presented antibacterial activity will act as bacteriostatic agents [25]. MIC is considered the standard parameter to determine the susceptibility of bacteria to an external agent like a compound or extract and corresponds to the lowest concentration of compound/extract that inhibits the bacterial grow. MBC is considered a relevant index of the bactericidal activity of antimicrobial agents. MBC corresponds to the lowest concentration of extract that reduces almost at 100% the viability of the bacteria. If the MIC and MBC are in the same range, the extract will probably kill the bacteria.
The antimicrobial activity of Z. zanthoxyloides polar extracts (ethanol and water extracts) against resistant isolates of S. aureus was previously reported by [16]. Similarly, the antibacterial activity of Z. leprieurii and Z. zanthoxyloides essential oils against a MSSA strain was reported by [26,27] published the results of the antibacterial activity of steam bark MeOH/H2O extract of Z. leprieurii against a MSSA strain. Those results sustained the ethnopharmacological properties described for these Zanthoxylum species, once they are mostly used for intestinal disorders and wound care.
Staphylococcus aureus strains are one of the major pathogens worldwide related with a large range of clinic manifestations with different levels of severity, triggering lethal infections, by synthesizing a large variety of toxins and enzymes. S. aureus strains developed an increasing antibiotic resistance such as a methicillin resistance (MRSA) and a vancomycin-intermediate resistance (VISA) [28]. The emergence of multidrug resistant bacteria strains and the increase of the pathogenicity of the strains are intimately related [29]. One approach to overcoming bacterial resistance mechanisms and restoring antibiotic efficacy is the use of inactive plant extracts when administered individually, in combination with antibiotics. This combinatory strategy may play an important role on the management of challenging infectious diseases [18].
In order to establish the potential interaction between the plants extracts and references antibiotics the checkerboard assay was performed as previously described. The extract that stood out was the Z. zanthoxyloides roots MeOH extract, which by itself presents no relevant antibacterial activity against resistant VISA strain (MIC= 500μg/mL) but was able to restore synergistically the antibacterial activity of the two antibiotics tested, amoxicillin from 60 to 4 μg/mL, (FICI = 0.18) and oxacillin from 125 to 7.5μg/mL (FICI = 0.31), corresponding in both cases to a 16- fold reduction. Z. leprieurii leaves AcOEt was also able to interact synergistically with oxacillin against the VISA strain, lowering the antibiotic MIC from 125 mg/mL to 7.5μg/mL (FICI = 0.31), corresponding to a 16-fold reduction.
For the MRSA strain (ATCC 43866) Z. leprieurii CH2Cl2 extract was able to revert synergistically the antibacterial activity of amoxicillin decreasing the MIC value from 60 to 4μg/mL (FICI= 0.46) corresponding to a 16-fold reduction. Z. leprieurii roots MeOH extract also displayed some synergistic interaction with amoxicillin against this strain, lowering the antibiotic MIC from 60 to 30μg/mL (FICI =0.5). The water extract of Z. zanthoxyloides roots when combined with oxacillin, decreased the antibiotic MIC value from 125μg/mL to 15μg/mL against MRSA strain (FICI=0.37).
Conclusion
Due to the growing problematic of drug-resistant bacteria it is urging to discover new compounds and new pathways to control and revert antibiotic resistance, to regulate the increasing bacterial derived diseases worldwide. The use of natural products as plant extracts reveal to have a multi target mechanism of action leading to a reduced prevalence of bacterial resistance. Therefore, the study of plant compounds and their synergistic interaction is a fundamental step in overcoming community health problems arising from current bacterial infections.
Some of the studied extracts were active against Gram positive strains but the results that stood out were the synergistic activity that some of the extracts presented, being able to restore the activity of the tested antibiotics. Overall, these results emphasize the worthwhile of additional studies of these species to better understand their efficacy and safety in traditional medicine and the mechanisms behind the restore of the antibacterial activity.
Acknowledgment:
This work was funded by national funds through FCT, under the project UIDB/00329/2020.
References
- Balunas MJ, Kinghorn AD (2005) Drug discovery from medicinal plants. Life Sci 78: 431-441.
- (2009) medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep10:194-200.
- Ríos JL, Recio MC (2005) Medicinal plants and antimicrobial J Ethnopharmacol 100(1-2): 80-84.
- O’ Neil J. Review on Antibiotic resistance. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Heal Wealth Nations, 2016; December 1-16. Review Paper - Tackling a crisis for the health and wealth of nations_1.pdf.
- Saleem M, Nazir M, Ali MS, Hussain H, Lee YS, et al. (2010) Antimicrobial natural products: An update on future antibiotic drug candidates. Nat Prod Rep 27(2): 238-254.
- Munita JM, Arias CA, Unit AR, Santiago A (2016) HHS Public Access Mechanisms of Antibiotic Resistance. HHS Public Access 4:1-37.
- https://www.who.int/news-room/detail/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis.
- Gupta PD, Birdi TJ (2017) Development of botanicals to combat antibiotic resistance. J Ayurveda Integr Med 8(4): 266-275.
- Efferth T, Koch E (2010) Complex interactions between phytochemicals. the multi-target therapeutic concept of phytotherapy. Curr Drug Targets 12(1): 122-132.
- Mundy L, Pendry B, Rahman M (2016) Antimicrobial resistance and synergy in herbal medicine. J Herb Med 6(2): 53-58.
- Van Wyk BE (2011) The potential of South African plants in the development of new medicinal products. South African J Bot 77(4): 812-829.
- Catarino L, Havik PJ, Romeiras MM (2016) Medicinal plants of Guinea-Bissau: therapeutic applications, ethnic diversity and knowledge transfer. J Ethnopharmacol 183: 71-94.
- Ouédraogo L, Fuchs D, Schaefer H, Kiendrebeogo M (2019) Morphological and molecular characterization of Zanthoxylum zanthoxyloides (Rutaceae) from Burkina Faso. Plants 8(9): 353-369.
- Bunalema L, Fotso GW, Waako P, Tabuti J, Yeboah SO (2017) Potential of Zanthoxylum leprieurii as a source of active compounds against drug resistant Mycobacterium tuberculosis. BMC Complement Altern Med 17(1): 89.
- Madureira AM, Ramalhete C, Mulhovo S, Duarte A, Ferreira M-JU (2012) Antibacterial activity of some African medicinal plants used traditionally against infectious diseases. Pharm Biol 50(4): 481-489.
- Cos P, Vlietinck AJ, Berghe DV, Maes L (2006) Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept.” J Ethnopharmacol 106(3): 290-302.
- CLSI (2008) M100-S18 Performance standards for antimicrobial susceptibility testing: 18th Informational Supplement. Clin Lab Stand Inst.
- Hemaiswarya S, Kruthiventi AK. Doble M (2008a) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15(8): 639-652.
- Madureira AM, Duarte A, Teixeira G (2012) Antimicrobial activity of selected extracts from Hakea salicifolia and H. sericeae (Proteaceae) against Staphylococcus aureus multiresistant strains. South African J Bot 81: 40-43.
- Wagner H, Bladt S (1996) Plant Drug Analysis: A Thin Layer Chromatography Atlas. 2nd ed. Springer- Verlag, Berlin.
- Patel K, Kumar V, Rahman M, Verma A, Patel DK (2018) New insights into the medicinal importance, physiological functions and bioanalytical aspects of an important bioactive compound of foods ‘Hyperin’: Health benefits of the past, the present, the future. Beni-Suef Univ J Basic Appl Sci 7(1):31-42.
- Mills-Robertson FC, Adjapong G, Appenteng M, Acheampong S, Asiedu-Larbi J (2016) Antimicrobial activities of six selected medicinal plants against Staphylococcus aureus. Am J Trop Med Hyg 95: 258-259.
- Ngane N, Biyiti L, Zollo A (2000) Evaluation of antifungal activity of extracts of two Cameroonian Rutaceae: Sabouraudia. J Ethnopharmacol 70 (3):335-342.
- Stavri M, Piddock LJ V, Gibbons S (2007) Bacterial efflux pump inhibitors from natural sources. J Antimicrob Chemother 59(6):1247-1260.
- Rodgers CJ (2001) Resistance of Yersinia ruckeri to antimicrobial agents in vitro. Aquaculture 196(3-4): 325-345.
- Tatsadjieu LN, Essia Ngang JJ, Ngassoum MB, Etoa FX (2003) Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloïdes and Zanthoxylum leprieurii from Cameroon. Fitoterapia 74(5): 469-472.
- Agyare C, Kisseih E, Yaa I, Poku P (2014) Medicinal plants used in wound care: Assessment of wound healing and antimicrobial properties of Zanthoxylum leprieurii. Issues Bio Sci Pharma Res 2: 81-89.
- Appelbaum PC (2007) Microbiology of Antibiotic Resistance in Staphylococcus aureus. Clin Infect Dis 45 Suppl 3: S165-170.
- Worthington RJ, Melander C (2013) Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol 31(3): 177-184.
- Ji HF, Li XJ, Zhang HY (2009) Natural products and drug discovery: Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? 10(3): 194-200.






























