A Validated Ultra-High-Pressure Liquid Chromatography Method for Separation of Sugammadex Related Substances and its Degradants in Drug Product
Kadir Aluc*, Gizem Kaya and Mine Gokalp
Abdi Ibrahim Pharmaceuticals, Abdi Ibrahim Production Facilities, Turkey
Submission: April 28, 2021; Published: May 14, 2021
*Corresponding author: Kadir Aluc, Abdi Ibrahim Pharmaceuticals, Abdi Ibrahim Production Facilities, Esenyurt, 34538 Istanbul, Turkey
How to cite this article:Kadir Aluc, Gizem Kaya, Mine Gokalp . A Validated Ultra-High-Pressure Liquid Chromatography Method for Separation of Sugammadex Related Substances and its Degradants in Drug Product. Glob J Pharmaceu Sci. 2020; 8(3): 555739. DOI: 10.19080/GJPPS.2021.08.555739.
Abstract
Ultra-high-pressure liquid chromatography (UPLC) is used for drug analysis in the pharmaceutical industry. Short turnaround time, method reliability, method sensitivity is the some of advantages of using UPLC. Sugammadex is a molecule that is modified cyclodextrin newly treatment for reversal neuromuscular blocking agent. This study aimed to develop and validate a new method, using UPLC to perform a quantitative analysis of Sugammadex related substances in the drug product. The separation in the described chromatographic system was accomplished using a mobile phase consisting of a mixture of phosphate buffer and acetonitrile and a UPLC BEH C8 column (50 x 2.1mm, 1.7μm) with gradient elution on a consistent flow rate of 0.4mL/min. PDA detection was carried out at a wavelength of 210nm. In line with The International Conference on Harmonization (ICH) guidelines the drug was exposed to various forced degradation conditions, including photolysis, oxidation, thermal degradation and hydrolysis under acidic, basic and neutral conditions. The linearity varied in the range from 0.9μg/mL to 11.0μg/mL for Sugammadex. The ranges of the detection and quantitation limits were determined to be 0.3μg/mL and 0.9μg/mL respectively for Sugammadex. The calculated correlation coefficients were found out to be higher than 0.999. The RSD values for repeatability and intermediate precision were determined to be lower than 2%. For all degration products of interest, the average recovery values were determined within the range of 95.00%-105.00%. We conclude that this newly developed analytical procedure applies to the quality control of drug product.
Keywords: Sugammadex; Related substances; Ultra high-pressure liquid chromatography; Degradants
Abbreviations: UPLC: Ultra-High-Pressure Liquid Chromatography; LOD: Limit of Detection; LOQ: Limit of Quantification
Introduction
Sugammadex molecule formula is Octakis-(6-S-(2-carboxyethyl)-6-thio)-cyclomaltooctaose octasodium salt with a modified y-cyclodextrin [1]. Cyclodextrins were for many years used excipients with solubilizing agents and stabilizing agents; however, its therapeutic properties were discovered at the end of the 20th century [2]. Sugammadex; a modified y-cyclodextrin derivative, approved by the European Medicines Agency in 2008 and by the U.S. Food and Drug Administration in 2016 [3,4]. Sugammadex is a very soluble in water, nontoxic and physiochemically stable molecule [2,5]. As one of the known uses of Sugammadex as a modified cyclodextrin, it reverses by creating a complex molecule with the rocuronium and vecuronium as neuromuscular blocking agents [6]. Vecuronium and rocuronium are used for anesthesia for during surgery in adults [7,8]. Sugammadex forms a complex with the neuromuscular blocking agents rocuronium or vecuronium in plasma and thereby reduces the amount of neuromuscular blocking agent available to bind to nicotinic receptors in the neuromuscular junction. The release of receptors encourages muscle movement [9,10]. The molecular encapsulation process occurs at a ratio of 1:1, and after when sugammadex is intravenously injected, it is bonding with neuromuscular agents and reducing their concentration in plasma [3,11,12]
Vecuronium bromide and rocuronium bromide are neuromuscular blocking medications that cause temporary paralysis and are especially useful for ventilation, general anesthesia, or tracheal intubation that patients may require for surgery [13]. Some clinical studies of neuromuscular blocking drugs; documented that incomplete recovery is associated with various side effects such as muscle weakness, airway obstruction, and respiratory complications [14,15]. Sugammadex provides a new treatment option to reverse the effects of those medications and possibly help patients quicker recovery and minimal side effects sooner post-surgery [8-16].
The molecular structure of Sugammadex and potential process/degradant related substances of Sugammadex are shown in (Figure 1). These related substances originate from the manufacturing process or degradation of Sugammadex. These related substances should be monitored according to ICH Q3B guideline [17]. Acceptance criteria are based on pharmaceutical studies or known safety data.
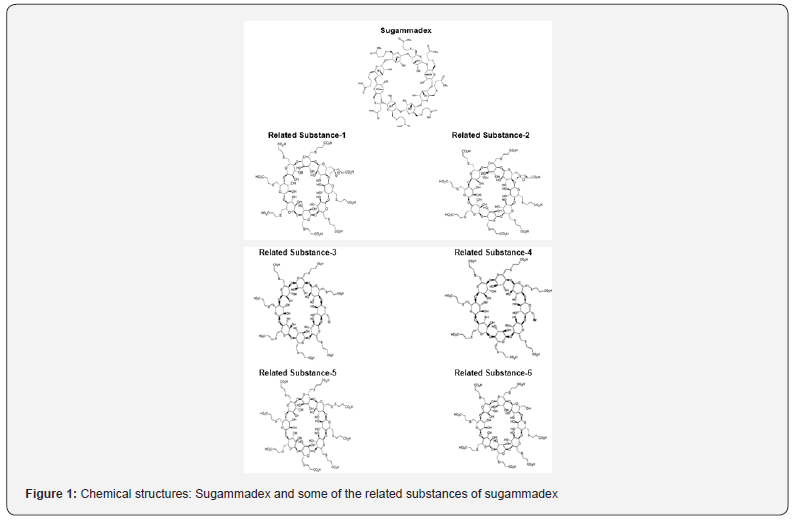
Related substance levels should be monitored and controlled by appropriate methods in order to prove that the drug product is within the specifications during the production and shelf life. In the literature, few analytical methods were reported using HPLC [18], UV spectrophotometer [19] and UPLC-TMS [20] for the assay of Sugammadex. However, based on the literature, pharmacopoeial review and currently available information, there is no method which can analyze the related substances of Sugammadex in the drug product.
Sugammadex assay methods presented in the literature which are performed by using HPLC gives the idea that similar conditions can give a lead to developing related substances methods for Sugammadex. Additionally, isolating and identifying the related substances in the least amount of time is not negligible for the process control monitoring of pharmaceutical industry. Hence, developing a more rapid and simple method would be useful and preferable. Ultra-high-pressure liquid chromatography (UPLC) would serve this purpose with its technical properties, which provides to increase the resolution power and also improve the sensitivity at the same time due to packing with sub-2 μm particles, analytes retain less time on the stationary phase [21]. Besides, reduced solvent consumption is another benefit compared to conventional HPLC [22]. In the view of such information, we identified and quantified six related substances by the developed UPLC method for 15 min of total analysis. UPLC revealed higher efficiency for determining the generated related substances from the force degraded samples.
Method validation for related substances was accomplished according to the ICH requirements, by carrying out forced degradation tests for the drug formula covering various conditions, photolysis, oxidation, hydrolysis and thermal degradation [23,24]. We anticipated that the results obtained out of the stability testing procedures would provide important contributions to decisionmaking processes for selecting proper packaging and specifically detect any potential degradants that may constitute during stability testing.
Experimental
Reagents
Sugammadex 100mg/ml solution for injection were produced in Abdi Ibrahim Pharmaceuticals, R & D center, in Istanbul, Turkey and obtained as a 100mg/mL stock solution in water. Working standards of Sugammadex and related substances was obtained from Dr. Reddy’s Laboratories Limited, India which is API supplier. All chemicals and solvents had analytical reagent grade. Acetonitrile was purchased from J. T. Baker Company. Other reagents such as phosphoric acid, hydrochloric acid, sodium hydroxide, and hydrogen peroxide were purchased from Merck.
Instruments
A reversed-phase UPLC system (Waters, USA) consisting of autosampler, pump, oven and UV/PDA detector was used. Data gathered and derived from chromatographic procedure was processed by the software of the Empower chromatography system.
Methods
The chromatographic separation was accomplished using an Acquity UPLC BEH C8 column (2.1 × 50mm, 1.7μm, Waters) maintained at 50°C. A solution of phosphate buffer/acetonitrile (95/5, v/v) containing 4.0mL of orthophosphoric acid in 2000mL milli-Q water and a solution of phosphate buffer/acetonitrile (15/85, v/v) containing 4.0 mL of orthophosphoric acid were used as mobile phase A and B, respectively. A linear mobile phase gradient with a flow rate of 0.4mL/min was selected, starting at 88% mobile phase A and raising mobile phase B content from 12% to 20% in ten minutes. Between ten and eleven minutes, the mobile phase B decreases back to 12% and the program is completed in 15minutes. The specific wavelength of 210nm was selected for detection. As diluent; phosphate buffer/acetonitrile (80/20, v/v) containing 4.0mL of orthophosphoric acid in 2000mL milli-Q water was used. It was then filtered with a 0.22μm membrane filter.
Standard solution preparation
9 mg of Sugammadex was weighed into 100mL volumetric flask accurately and the volume was completed to final volume of 100ml with water. Further, 1mL of resulting solution was diluted in 10mL of the volumetric flask with water. The standard solution was kept at 25oC and were stable for a 100hour
Sample Preparation
Sugammadex 100mg/ml solution for injection was prepared at Abdi Ibrahim Pharmaceuticals, R & D center, in Istanbul, Turkey. An accurate amount of drug product 1ml was weighed and transferred into a 20mL volumetric flask and the volume was completed to final volume of 20mL with diluent. The flasks were carefully shaken.
Results
Method validation
The analytical method development and validation were carried out in line with ICH guidelines. The methods were validated based on the parameters covering system suitability, accuracy, precision, specificity, linearity, range, limit of detection and limit of quantification [25].
Specificity/Forced degradation
Specificity is the ability of the method to measure the analyte response in the presence of all related substances. For specificity determination, all related substances were prepared individually and injected into UPLC to confirm the retention times. Retention time, relative retention time according to Sugammadex and resolution information are presented (Table 1). A solution of a blank, placebo, sample, spiked sample (sample spiked with all related substances) were prepared and injected into UPLC to check existence of any interference with analyte peaks from blank, placebo and any degradation peaks. From the injections of spiked sample, it was confirmed that the known related substances peaks were well separated from each other. There was no interfering peak at the retention time of Sugammadex and known related substances peaks in chromatogram of blank and placebo. A typical representative chromatogram is shown in figure 2.
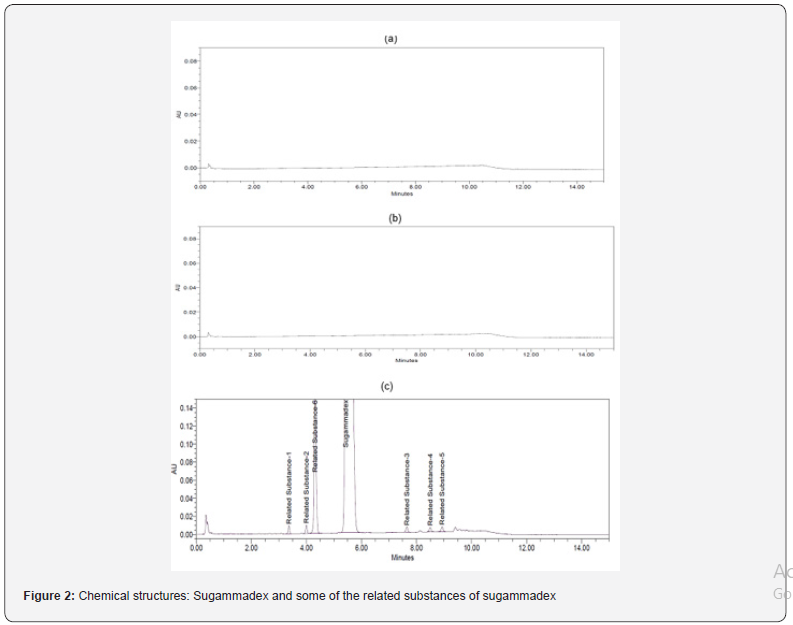
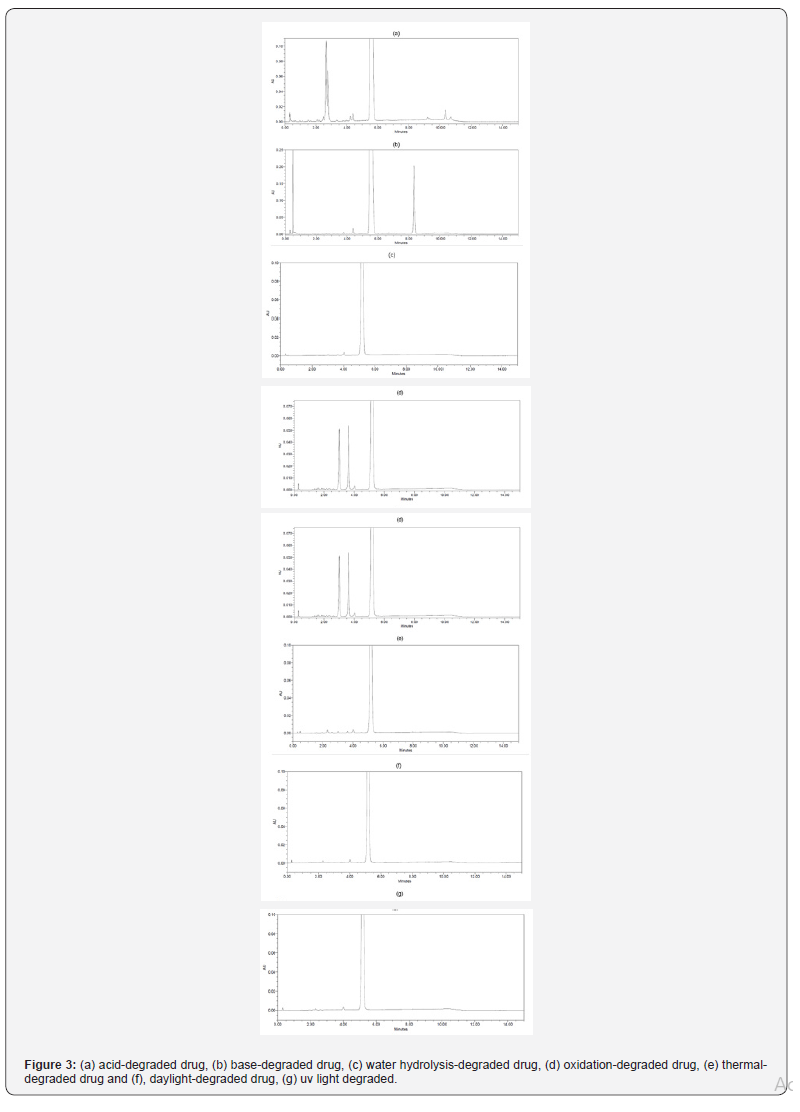
Forced degradation investigation was carried out in line with ICH guidelines Q1A (R2) to show the stability-defining characteristic and specificity of the developed analytical method. The drug product was prepared as a sample and investigated under various forced degradation conditions described as follows; under alkaline conditions (5N NaOH at ambient temperature during 2hours), acidic conditions (5N HCl at ambient temperature during 6hours), neutral conditions (at 40°C±%75RH during 1 week), oxidative conditions (0.024% v/v H2O2 at ambient temperature during 6 hours). The samples from alkaline and acidic degradation conditions were neutralized using adequate amount of 5N HCl and 5N NaOH, respectively, and completed to the final volume with the diluent. Thermal forced degradation conditions were created by keeping the investigational medicinal product in heat-controlled oven at 80°C during a 2 day. The Sugammadex drug product was exposed to UV (200 W/m2) and day-light lamp for (1.2million lux) to check photostability. Upon completion of pre-defined time, the solutions resulting from forced degradation condition testing were diluted with the diluent and the samples for degradation testing were subject to analysis by the developed UPLC method as mentioned in the chromatographic condition section. A Photodiode Array (PDA) detector was used to define peak purity concerning peaks resulting from all samples from forced degradation condition testing. Purity has been met under all forced degradation conditions for related substances of Sugammadex.
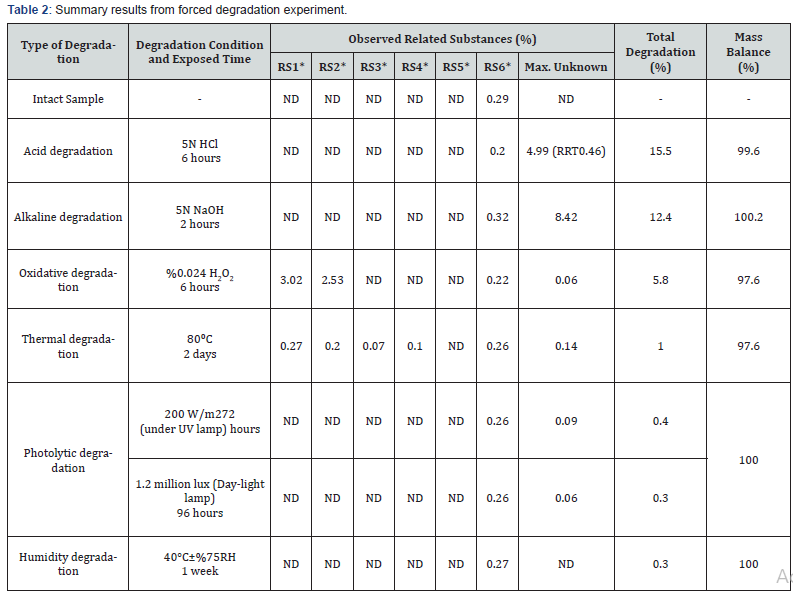
The summary of results obtained from forced degradation experiment results is presented in (Table 2). It was determined that when Sugammadex drug product is exposed to acid degradation, alkaline degradation and oxidative degradation, an increase was observed at some of known related substances and unknown related substance results which provides obtaining mass balance by compensating the decrease at assay results. Typical chromatograms showing the degradation conditions are presented in figure 3.
LOD and LOQ
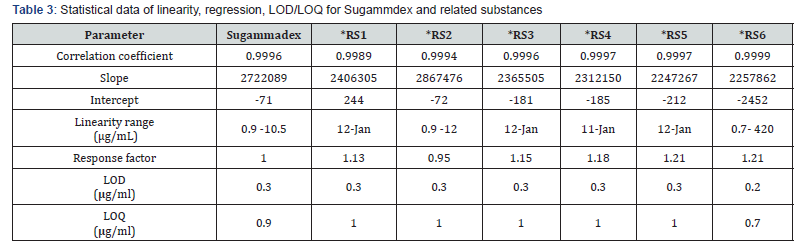
LOD and LOQ values were determined based on the standard deviation of the response and the slope approach according to ICH. Sugammadex and related substances solutions were injected into UPLC from lower concentration to higher concentration in the range of 0.9 –12μg/mL for Sugammadex, related substance 1,2,3,4,5 and 0.7–420μg/mL for related substance 6, respectively. A plot of peak area response versus concentration was drawn, and LOD/LOQ values were predicted by residual standard on deviation response (SD) and slope (S) method using the formula 3.3 × SD/S for LOD and 10 × SD/S for LOQ. For LOD and LOQ evaluation, the solutions of Sugammadex and all related substances were prepared at obtained concentration levels and confirmed by analyzing two and six replicate injections, respectively. The LOD and LOQ values are presented in (Table 3).
Linearity
The linearity of the method was determined by taking the same data obtained for LOD and LOQ. The linearity of Sugammadex and all related substances at different concentrations were studied in the range of LOQ to 12μg/mL (120 %) for related substance1,2,3,4,5 and LOQ to 420μg/mL (120 %) for related substance 6, respectively. The data were subjected to statistical analysis using a linear regression model. The statistical parameters such as slope, intercept and correlation coefficient values were calculated. The correction factors for related substances and Sugammadex were evaluated by slope (S) method using the formula: slope of the Sugammadex standard/slope of the related substance. The calculated statistical results are presented in (Table 3).
Precision
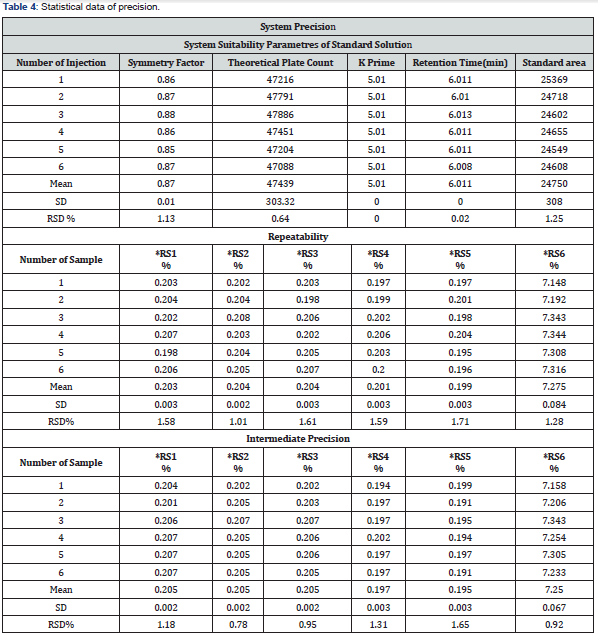
The precision of the method was evaluated with replicate injections of standard and sample solutions. Six consecutive injections from standard solution were performed for system precision. RSD% between the peak areas obtained from injections were calculated. The repeatability and intermediate precision of the method was studied by analyzing six sample solutions separately by adding related substances at known concentration levels. The precision results are presented in (Table 4).
Accuracy
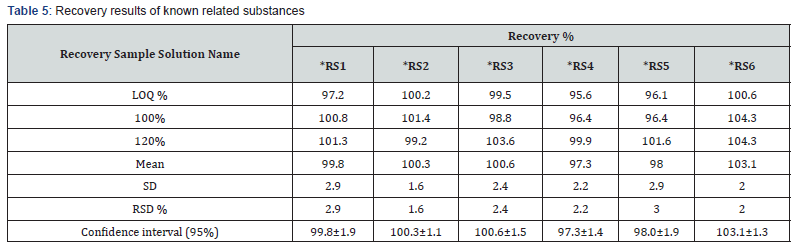
Accuracy experiment was performed by adding all related substances at three different levels of LOQ, 100% and 120% into Sugammadex drug product. Recovery study was performed by adding of related substances into Sugammadex drug product. Samples were prepared in three replicates, analyzed and the percentage recoveries were calculated. The results are presented (Table 5).
Robustness
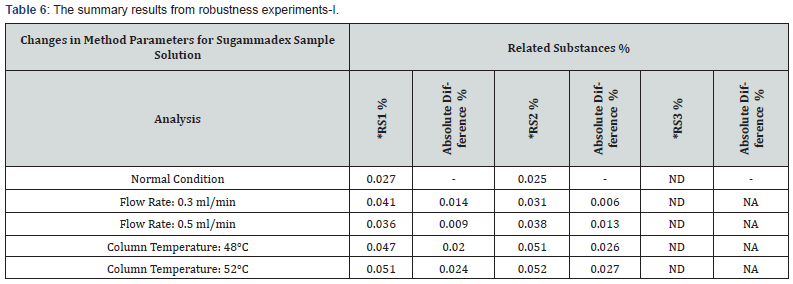
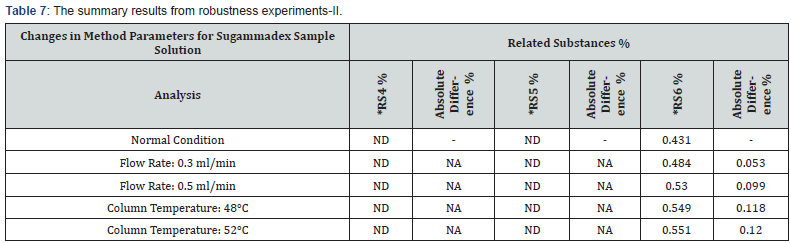
To assess the robustness, the chromatographic conditions were deliberately altered. In each robustness condition, blank, placebo, standard, sample solutions were prepared and the results were compared to repeatability results. The absolute difference between results of repeatability and robustness parameters means of related substances were evaluated. It was observed that there was not too much variation in the result. It is determined that this method is robust to the small variation in UPLC conditions, i.e., flow by ±0.1ml/min (with respect to 0.4ml/ min) and initial column temperature by ±2°C (with respect 50°C). The summary results from robustness experiments are presented in table 6 & 7.
Discussion
Method development and optimization
Initially, the method was developed on HPLC for the determination of related substances of Sugammadex in the drug product. Due to the longer run time of about 70min, the HPLC method was not validated but further adapted to UPLC wherein the run time was reduced about four times from 70 to 15min. The core objective of developing the UPLC method was to detect all six related substances and achieve the separation of them in a short time with high sensitivity and less reagent consumption.
UPLC Column Selection
Based on parameter-like resolution, tailing factor, and selectivity especially for all related substances, Acquity UPLC BEH C8 column (2.1 × 50mm, 1.7μm) was selected for analysis.
Buffer selection
Various buffers like phosphate and acetate with pH ranging from 2.0 to 5.0 were tried for development. It was observed that at acidic pH of about 2.0 which is equivalent to the pKa of the phosphate buffer, the separation and the tailing factors are satisfactory. Therefore, phosphate buffer was selected for analysis. It was sufficient to perform pH control in the mobile phase mixture.
Organic modifier selection
Organic modifiers like acetonitrile and methanol were evaluated as mobile phase A and B. Appropriate chromatographic separation was achieved when acetonitrile was used as mobile phase A and B in terms of better resolution and tailing factor. When methanol was tried as an organic modifier, the tailing factor for all the peaks was not within the column performance criteria. Therefore, acetonitrile was finalized as organic modifier based on system suitability parameters-like resolution, tailing factor, and selectivity.
Optimization of chromatographic conditions
Various combinations of gradient time programs were tried and then finalized wherein all related substances are well separated from the main peak due to Sugammadex. The wavelength for analysis was selected as 210 nm which is UV maxima of Sugammadex. The rationale for selecting the flow rate of 0.4ml/min is based on system suitability parameters-like resolution, tailing factor, and selectivity.
Conclusion
In this paper, we have presented a newly developed and validated UPLC method to determine and quantify some of the related substances of Sugammadex in the drug product. We evaluated this method for linearity, precision, accuracy, LOD, LOQ, selectivity and robustness. The selectivity of the method is evaluated as per ICH guidelines by carrying out forced degradation tests of Sugammadex solution for injection. Accordingly, under applied forced degradation conditions decrease in chromatographic purity results is compensated with an increase in related substances results and mass balance is provided. The outcomes of these tests demonstrated that the method was stable based on the findings of Sugammadex and their related substances. Thereby, we concluded that the newly developed and validated method proposed in this current article is time saving, provides less solvent consumption, has satisfactory resolution and could be widely used in the routine practice to simultaneously determine Sugammadex related substances in drug product.
References
- Mohamed Naguib, Sorin J Brull (2009) Sugammadex: A novel selective relaxant binding agent. Expert Rev Clin Pharmacol 2(1): 37-53.
- Susana Santos Braga (2019) Cyclodextrins Emerging Medicines of the New Millennium. Biomolecules 9(12): 801.
- Yutaka Murata, Shuji Kawamoto, Kazuhiko Fukuda (2020) Rocuronium Has a Suppressive effect on Platelet Function via the P2Y12 Receptor Pathway In Vitro That Is Not Reversed by Sugammadex. Int J Mol Sci 21(17): 6399.
- European Medicines Agency, Bridion (sugammadex), (2008) EPAR summary for the public.
- Keating GM (2016) Sugammadex A Review of Neuromuscular Blockade Reversal. Drugs 76(10): 1041–1052
- Sergey V Kurkova, Martin Messnera, Marjoleine Lucassenb, Diels J van den Dobbelsteenc, John den Engelsmanb, et al. (2011) Evaluation of sugammadex self-association. Int J Pharm 413(1-2): 134-139.
- Skyler Lentz, Katelin M Morrissette, Blake A Porter, Kyle M DeWitt, Alex Koyfman, et al. (2020) What is the role of Sugammadex in the emergency department? The Journal of Emergency Medicine 60(1): 44-53.
- Lajos Szente, Julianna Szem´an, Tam´as Sohajda (2016) Analytical characterization of cyclodextrins: History, official methods and recommended new techniques. J Pharm Biomed Anal 130: 347-365.
- Hans D de Boer, Jan van Egmond, Jacques J Driessen, Leo HD Booij (2007) Update on the management of neuromuscular block: focus on sugammadex. Neuropsychiatr Dis Treat 3(5): 539-544.
- Chryssoula Staikou, Martina Rekatsina (2017) Use of rocuronium and sugammadex under neuromuscular transmission monitoring in a patient with multiple sclerosis. Saudi J Anaesth 11(4): 472-475.
- Stefan Josef Schaller, Heidrun Lewald (2016) Clinical pharmacology and efficacy of sugammadex in the reversal of neuromuscular blockade. Expert Opin Drug Metab Toxicol 12(9): 1097-1108.
- Thomas M Hemmerling, Cedrick Zaouter, Goetz Geldner, Dirk Nauheimer (2010) Sugammadex a short review and clinical recommendations for the cardiac anesthesiologist. Ann Card Anaesth 13(3): 206-216.
- Alen Mathew, Anish NG Sharma, P Ganapathi, P Shankaranarayana, M Nazim, et al. (2016) Intraoperative hemodynamics with vecuronium bromide and rocuronium for maintenance under general anesthesia. Anesth Essays Res 10(1): 59-64.
- Michele Carron, Francesco Bertoncello, Giovanna Ieppariello (2017) Profile of sugammadex for reversal of neuromuscular blockade in the elderly: current perspectives. Clin Interv Aging (13): 13-24.
- Hongwei Ren, Huangwei Lv (2016) Neuromuscular Effects of Rocuronium Bromide in Patients in Statin Therapy for at least Three Months. Basic Clin Pharmacol Toxicol 119(6): 582-587.
- Mark Welliver, Dennis Cheek, Juergen Osterbrink, John McDonough (2015) Worldwide experience with sugammadex sodium: implications for the United States. AANA J 83(2): 107-115.
- ICH Harmonised Tripartite Guideline. Q3A(R2) Impurities in new drug.
- Hemangi Parekh, Parin Chokshi, Rajashree Mashru (2020) Analytical Method Development and Validation for the Estimation of Sugammadex. Journal of Drug Delivery & Therapeutics 10(1): 52-59.
- Ashok Chakravarthy V, Sailaja BBV, Praveen Kumar A (2017) Method development and validation of ultraviolet-visible spectroscopic method for the estimation of assay of sugammadex sodium, apremilast, riociguat and vorapaxar sulfate drugs in active pharmaceutical ingredient form. Asian Journal of Pharmaceutical and Clinical Research 10(2): 241-250.
- Zeng W, Xu Y, Constanzer ML, Goykhman D, Woolf EJ (2018) Determination of Sugammadex in Human Plasma using Protein Precipitation and Ultra High-performance Liquid Chromatography/Tandem Mass Spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 1(1): 1007.
- Michael W Dong, Kelly Zhang (2014) Ultra-high-pressure liquid chromatography (UHPLC) in method development. TrAC Trends in Analytical Chemistry 63: 21-30.
- Emma Johansson, Anders Karlsson, Jufang Wu Ludvigsson (2020) Ultra high-performance liquid chromatography method development for separation of omeprazole and related substances on core-shell columns using a Quality by Design approach. Journal of separation science 43(4): 696-707.
- ICH Harmonised Tripartite Guideline. Q1A(R2) Stability testing of new drug substances and products.
- ICH Harmonised Tripartite Guideline. Q1B Stability testing: Photostability testing of drug substances and products.
- ICH Harmonised Tripartite Guideline. Q2(R1) Validation of analytical procedures: Text and Methodology.






























