Analytical Method Development and Validation of UV-Visible Spectrophotometric Method for the Estimation of Saxagliptin in Gastric Medium
Deepika Joshi1*, Bhavana Singh1, Archana Rautela2 and Nidhi Semwal1
1School of Pharmaceutical Sciences, India
2Gyani Inder Singh Institute of Professional Studies, India
Submission: March 24, 2021; Published: April 22, 2021
*Corresponding author: Deepika Joshi, School of Pharmaceutical Sciences, SGRRU, India
How to cite this article:Deepika J, Bhavana, Archana R, Nidhi S. Analytical Method Development and Validation of UV-Visible Spectrophotometric Method for the Estimation of Saxagliptin in Gastric Medium. Glob J Pharmaceu Sci. 2020; 8(2): 555735. DOI: 10.19080/GJPPS.2021.08.555735.
Abstract
Aim: A simple, accurate, precise, cost effective, rapid and sensitive UV/visible spectrophotometric method was developed for the determination of Saxagliptin in active pharmaceutical dosage form. The developed method was validated as per ICH guidelines. Method: The purity of saxagliptin was characterized by solubility profile, melting point, Fourier Transform Infra-Red. The drug was analyzed using UV/visible spectrophotometric method was validated in terms of linearity, accuracy, precision, specificity, Limit of detection and Limit of quantitation. The solvent used was methanol: water (15:85, v/v) and the wavelength corresponding to maximum absorbance of the drug were found at 204 nm. Result: Melting point of drug was found 101°C nearly corresponds to its actual melting range. The linear response for concentration range of 2-10 μg/ml of saxagliptin was recorded with y = 0.1126x - 0.0103, regression coefficient r2 = 0.99068. The accuracy was found between 93.75- 104.16%. Precision for intra-day and inter-day was found to be within the limits. To establish the sensitivity of the method, limit of detection (LOD) and limit of quantification (LOQ) were determined which were found to be 6.77 μg /mL and 20.33 μg /mL respectively. Conclusion: The drug was confirmed by interpretation of UV spectra. Hence proposed method stands out validated and thus may be used for routine analysis of Saxagliptin in pharmaceutical dosage forms.
Keywords: Spectrophotometric method; Saxagliptin; Methanol; Water; Melting point
Abbreviations: UV spectroscopy: Ultraviolet Spectroscopy; ICH: International Conference on Harmonisation; DPP: Dipeptidyl Peptidase; FTIR: Fourier Transform Infra-Red; LOD: Limit of Detection; LOQ: Limit of Quantification
Introduction
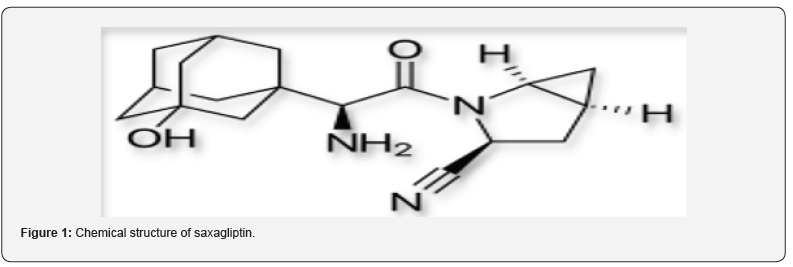
Saxagliptin (SXG) is chemically (1S, 3S, 5S)-2- [(2S)-2-Amino-2-(3 hydroxytricyclo [3.3.1.13,7] dec- 1-yl) acetyl]-2-azabicyclo [3.1.0] hexane-3-carbonitrile previously identified as BMS-477118 as shown in figure 1. The empirical formula is C18H25N3O2.H2O, and the molecular weight is 333.43 [1-4]. Saxagliptin is an oral hypoglycemic or anti-diabetic drug of the dipeptidyl peptidase-4 (DDP-4) inhibitor class. The inhibition of DPP-4 increases levels active of glucagon like peptide 1 (GLP-1), which inhibits glucagon production from pancreatic alpha cells and increases production of insulin from pancreatic beta cells. In 2009, U.S. Food and Drug Administration (FDA) approved saxagliptin and sold under the brand name Onglyza. In adults with type-2 diabetes mellitus, saxagliptin is suggested as an add-on to diet and exercise to improve glycemic control [5-11].
Literature survey reveals that the drug can be estimated only by LC-MS/MS, spectrophotometric method have been reported [12,13]. The aim of the present work was to develop a simple, sensitive, precise and accurate UV/Visible spectrophotometric method for the determination of saxagliptin in its pure form and pharmaceutical formulations further, to validate the developed method.
Materials and Methods
Chemical and reagents
All the chemicals used were of analytical grade. All the solutions were freshly prepared in Methanol and 0.1N HCl (15:85, v/v). Authentic of saxagliptin were obtained as gift samples from Mylan Laboratories Limited, Hyderabad
Methods
Saxagliptin was estimated using UV-Visible spectrophotometer (Shimadzu model 1800 double beam) at the wavelength of maximum absorption (204 nm) in acidic medium containing 0.1N HCl. The drug was characterized by solubility, melting point, and Fourier Transform Infra-Red (FTIR) techniques. The analysis of the drug was carried out by UV-Visible method which was validated analytical parameters like linearity, precision, and accuracy as per guidelines laid down by International Conference on Harmonization (ICH).
Physical characterization of saxagliptin
The physical characterization of procured drug sample of saxagliptin was determined on the basis of following parameters.
Organoleptic properties
The organoleptic properties have been determined for nature, colour, taste and odour of the pure sample of saxagliptin
Solubility
Excess amount of saxagliptin was dissolved in 10 ml distilled water till a saturated solution was obtained. The saturated solution of saxagliptin was stirred for 48 hrs on magnetic stirrer at 100rpm and room temperature (at 25 ± 1ºC). Then the sample was centrifuged for 10 min at 10,000 rpm. Clear supernant was collected using 0.22 μm syringe filter and analysed using UV spectrophotometer. The results were analysed and noted [14].
Melting point
Melting point of saxagliptin was determined using capillary melting point apparatus. In this method a small quantity of drug was filled in a capillary tube open both the ends and it was placed along with thermometer in melting point apparatus.
Fourier transform Infrared spectroscopy (FTIR)
FTIR spectrum was used as an analytical technique for identification of pure drug sample by KBr method using FTIR spectrometer (Agilent Technologies, USA). Peaks of individual pure drug were compared with reference FTIR peaks. Samples were previously prepared with KBr at 1: 5 (sample: KBr, w/w). KBr disks were prepared by compressing the powders at a pressure of 5 tons for 3 min in a hydraulic press and scanned against a blank KBr disk at wave numbers ranging from 500 to 4000 cm-1 with a resolution of 1.0 cm-1 [15].
Analytical method for drug concentration measurements (UV/Visible method)
The ultraviolet spectrophotometric method was selected for the estimation of saxagliptin in the range of 200 to 400 nm and the λmax was determined.
Diluent preparation
Methanol and 0.1N HCl (15:85, v/v) were used as a diluents.
Preparation of saxagliptin stock solution (20μg/ml) in 0.1N HCl
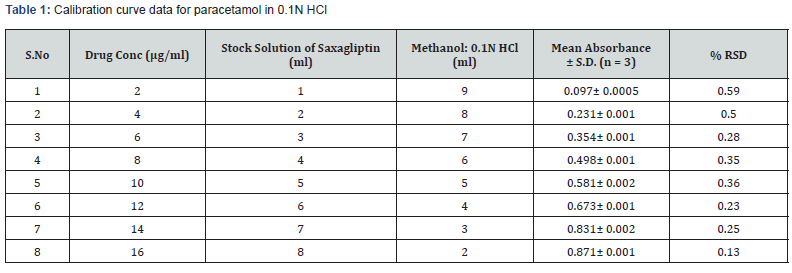
Saxagliptin was weighed equivalent to 100mg and transferred into 100ml volumetric flask then 15ml of methanol was added and shaked well to dissolve it after that the volume was made up to 100ml with 0.1N HCl. From that 2ml of solution was withdrawn and taken in 100ml volumetric flask. The volume was adjusted with diluent up to up to 100ml with 0.1N HCl [16,17] as shown in table 1.
Selection of detection wavelength
Drug solution was scanned over the range of 200- 400 nm. The wavelength of saxagliptin was determined to be 204 nm.
Preparation of working standard
Prepare a series of dilute solution from the above stock solution according to table 2 in 10 ml volumetric flask. Measure the absorbance of the solutions at 204 nm using distilled water as a blank. Plot a graph by taking concentration (μg/ml) on X-axis and absorbance on Y-axis. This plot gives a straight line and the linearity can be determined using y = mx + C formula. From the calibration curve calculate the Coefficient of determination R2 value, slope m and intercept C using the following formula, OR by excel sheet.
Construction of calibration curve
Pipette out 1,2,3,4,5,6,7, and 8 ml of working solution and transfer into separate 10 ml volumetric flasks. Dilute all of them to 10 ml with water to get solution of concentrations to 2, 4, 6,8,10,12,14,16 μg/ml respectively
UV Method Validation
The ultraviolet spectrophotometric method was validated for accuracy, precision, linearity, detection limit, quantitation limit and robustness [18-20].
Linearity
Appropriate aliquots of saxagliptin working standard solutions were taken in different 10 ml volumetric asks and diluted up to the mark with distilled water to obtain final concentrations of 2, 4, 6, 8, 10 μg/ml. Calibration curves were constructed by plotting absorbance versus concentrations and regression equations were calculated for both the drugs.
Range
The Range of the analytical method was decided from the interval between the upper and lower level of the calibration curve by plotting curve.
Precision
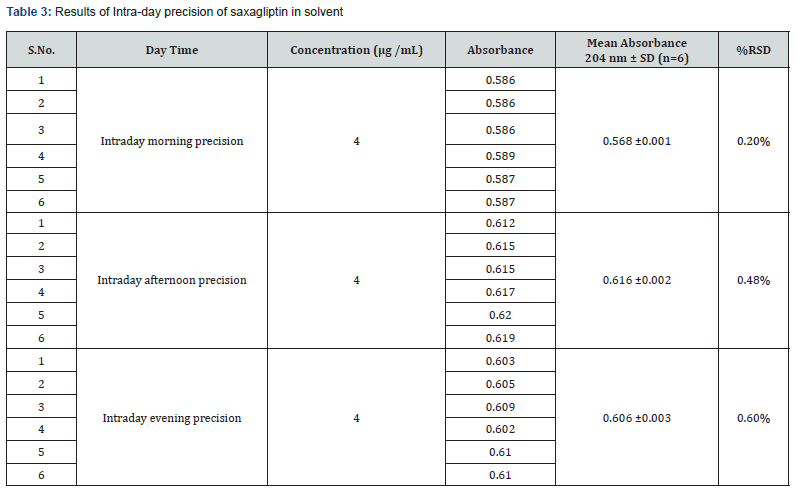
Intraday precision was determined by analyzing the drugs at concentrations (4μg/mL) and each concentration for three times, on the same day. Inter-day precision was determined similarly, but the analysis is carried out daily, for two consecutive days. Repeatability (intraday) of the method was determined by analyzing six samples of the same concentrations of the drug (4μg/mL). The absorbance of each was measured and reported in terms of relative standard deviation to obtain the variation.
Accuracy
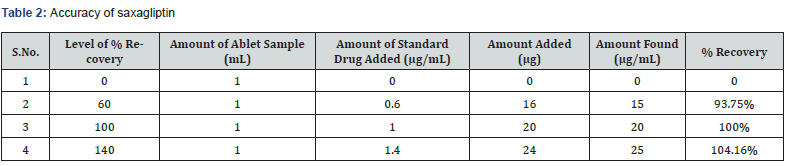
The accuracy of the method was determined by calculating recoveries of saxagliptin by method of standard additions at three different levels 60, 100 and 140 %. Mean percentage recovery was determined. Recovery values were calculated and shown in table 1.
Detection limit
The Detection Limit of an individual analytical procedure is the lowest amount of analyte in a sample which can be detected but not necessarily quantitated as an exact value. The detection limit (LOD) may be expressed as. LOD= 3.3σ/S Where σ = Relative standard deviation of the response. S = the slope of the calibration curve (of the analyte).
Quantitation Limit
The Quantitation limit of an analytical procedure is the lowest amount of analyte in a sample, which can be quantitatively determined with suitable precision and accuracy.
Quantitation Limit (LOQ) may be expressed as: LOQ = 10σ/S Where
σ = Relative standard deviation of the response.
S = the slope of the calibration curve (of the analyte).
Results & Discussion
Organoleptic properties
Saxagliptin was found to be white colored, non-hygroscopic, crystalline powder.
Solubility
The solubility of saxagliptin in water was found to be 2.12 ± 0.28 mg/L corresponding to reference value of 2.9 mg/L.
Fourier transform infrared spectroscopy (FTIR)
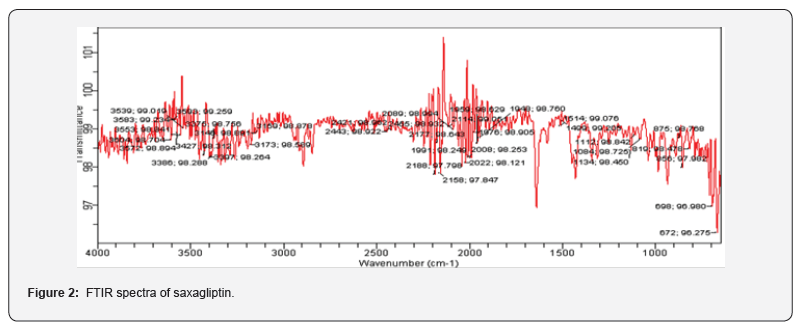
FTIR spectrum for identification of pure drug sample was done by FTIR spectrometer. Major absorption peaks of saxagliptin was found at 3450.12 cm-1 (N-H stretching), 2912.61 cm-1 (C-H stretching), 3301 cm-1 (OH stretching), 1614.47 cm-1 (C-N stretching), 1255.70 cm-1 (C-O stretching) as shown in figure 2 which corresponds to the literature peaks confirming the purity of drug sample.
Melting point
The melting point was determined by capillary melting point apparatus. The observed melting point of saxagliptin is 101°C
Analytical method for drug concentration measurements (UV/VIS Method)
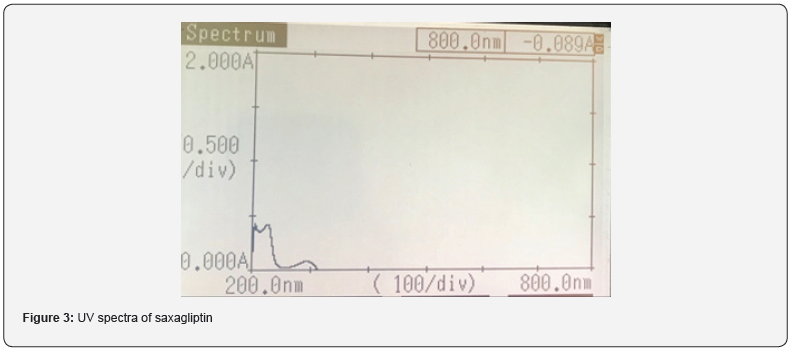
Selection of detection wavelength: The wavelength of saxagliptin was determined to be 204 nm as shown in figure 3.
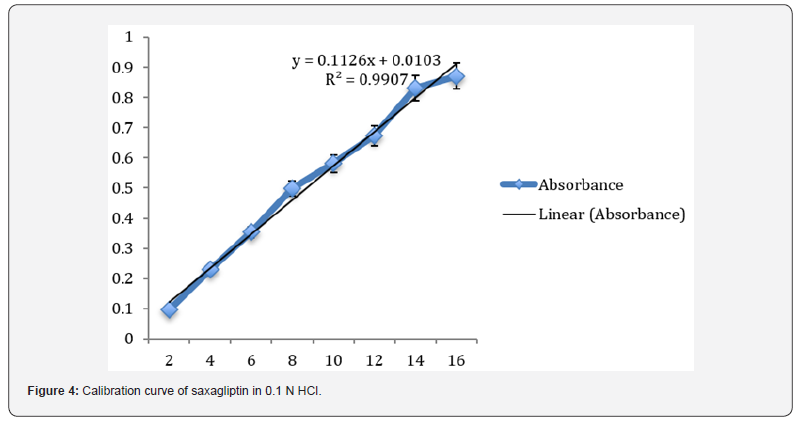
Preparation of standard plot for saxagliptin: Absorbance of the resultant solution was measured at 204 nm using blank. A graph was plotted between the concentrations and their respective absorbance. The response of the drug was found linear in the entire investigational range of 2 to 10μg/ml as shown in table 1. The calibration curve showed the linear equation as, y = 0.1126x - 0.0103, with a correlation coefficient, r2 = 0.99068, where y represents absorbance (optical density) and x represents the concentration (μg /ml) as shown in figure 4.
Method validation: The developed method was validated as per ICH guidelines for the following parameters:
Linearity: The linearity for Saxagliptin was found to be linear in the range of 2-10 μg/ml. The regression equation was found to be y = 0.1126x - 0.0103, r2 = 0.99068.
Range: The observed range of saxagliptin in test solution was observed from 0.097± 0.0005 to 0.871± 0.001.
Accuracy: The accuracy of the analytical method for Saxagliptin was determined at 60%, 100% and 140% levels of standard solution. Absorbance was measured at 204 nm and results were expressed in terms of % recoveries in table 2.
Precision: The Intra-day and Inter-day precision were carried out using same optimized conditions. The precision (measurement of inter-day, intra-day repeatability) results showed good reproducibility with the relative standard deviation (% RSD) below 2.0 % as shown in table 3 & 4 respectively. This indicated that the method was highly precise.

Limit of detection (LOD) and limit of quantification (LOQ): LOD and LOQ of method were determined to be 6.77 μg /mL and 20.33 μg /mL respectively. LOD and LOQ indicate that method was highly sensitive and fast.
Conclusion
The method was validated and found to be simple, sensitive, accurate and precise as per ICH guidelines [21]. The % RSD for the validation parameters was found to be less than 2%. Hence proposed method may be used for routine analysis of these drugs in pharmaceutical dosage forms. Accuracy of proposed method was confirmed by performing accuracy studies that showed the results within the range. Precision of proposed UV method was confirmed by performing intra-day and inter-day precision. Results were well within acceptance criteria that indicate excellent scope of the method for the determination of Saxagliptin in pharmaceutical dosage forms and bulk.
Acknowledgment
All authors have contributed significantly in the preparation of the manuscript and are in agreement with the content of the manuscript and agree to submission to Global Journal of Pharmacy & Pharmaceutical Sciences, Juniper Publishers. Authors are grateful to Mylan Laboratories Limited, Hyderabad for providing the gift sample. The authors express gratitude to School of Pharmaceutical Sciences, SGRRU, Dehradun.
Conflict of Interest
The authors declare that there is no conflict of interest that could be prescribed as prejudging the impartiality of the review. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding Source
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Onglyza Product information (2009) Bristol-Meyers-Squibb. Princeton New Jersey, USA.
- Australian Drug Evaluation Committee Recommendations (2010) Australian Government Department of Health and Aging.
- Deacon CF, Holst JJ (2009) Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther 26(5): 488-499.
- Robertson JG (2005) Discovery and preclinical pro le of saxagliptin (BMS-477118): A highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 48(15): 5025- 5037.
- Deanna KS, Jasmine GD, Zachary WA, Kulasa K, Edelman S (2010) Saxagliptin: the evidence for its place in the treatment of type 2 diabetes mellitus. Core evidence 21(5): 23-37.
- Tahrani AA, Piya MK, Barnett AH (2011) Saxagliptin: A New DPP-4 Inhibitor for the treatment of Type 2 diabetes mellitus. Adv Ther 26(3): 249-262.
- Carolyn DF, Jens HJ (2011) Saxagliptin: A new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther 26(5): 488-499.
- Singh S, Sethi S, Khanna V, Benjamin B, Kant R, Sattigeri J, et al. (2011) A potent, selective and slow-binding inhibitor of dipeptidyl peptidase-IV for the treatment of type 2 diabetes. Eur J Pharmaco 652(1-3): 157-163.
- Sherwyn L, Schwartz MD (2010) Treatment of Elderly Patients with type 2 diabetes mellitus: A Systematic Review of the Benefits and Risks of Dipeptidy l Peptidase-4 Inhibitors. Amer J Geria Pharmacothera 8(5): 405-418.
- Hollander P, Jia L, Allen E, Che R (2009) Saxagliptin Added to a Thiazolidinedione Improves Glycemic Control in Patients with type 2 diabetes and Inadequate Control on Thiazolidinedione Alone. J Clin Endocrinol Metab 94(12): 4810-4819.
- Yang W, Pan C, Tou C, Zhao J, Nilsson IG (2011) Efficacy and safety of saxagliptin added to metformin in Asian patients with type 2 diabetes mellitus: A randomized controlled trial. Dia Res Clin Pra 5 3(1): 1-8.
- Hess C, Musshoff F, Madea B (2011) Simultaneous identi cation and validated quanti cation of 11 oral hypoglycaemic drugs in plasma by electrospray ionisation liquid chromatography–mass spectrometry. Anal Bioanal Chem 400(1): 33-41.
- Kalaichelvi R, Jayachandran E (2011) Validated Spectroscopic method for the estimation of Saxagliptinin pure and from tablet formulation. Int J Pharm Pharm Sci 3: 179-180.
- Thadkala K, Nanam PK, Rambabu B, Sailu C, Aukunuru J (2014) Preparation and characterization of amorphous ezetimibe nanosuspensions intended for enhancement of oral bioavailability. Int J of Pharm Inv 4(3): 131-137.
- Sharma D, Bhavna (2019) Formulation and Evaluation of Polymeric Nanomicelles of Gliptin for Controlled Drug Delivery. Drug Delivery Letters 9(2): 146-156
- Beckett AH, Stenlake JB (2004) Pharmaceutical Chemistry part 2. CBS publisher and distributor, pp. 284-286.
- Patel K, Patel A, Dave J, Patel C (2012) Absorbance correction method for estimation of telmisartan and metoprolol succinate in combined tablet dosage forms. Pharm Methods 3(2): 106-111.
- ICH Q1A (R2) (2003) Stability Testing of New Drug Substances and Products.
- Konari SN, Jacob JT (2015) Application of Analytical Validated High-Performance Thin-Layer Chromatographic Technique for the Multicomponent Analysis of Cardiovascular Drug Combos in Pharmaceutical Dosage Form. J P C 28(5): 354-361.
- ICH Q2 (R1) (2005) Validation of Analytical Procedures: Text and Methodology.
- ICH Harmonised Tripartite Guideline (1994) Text on Validation of Analytical Procedures. International Conference on Harmonization, Geneva, Switzerland, p. 1-5.






























