Alcohol-Induced Dose Dumping Studies in Gastro-Resistant Hard Gelatine Capsules Containing Immunosuppressant Drug
Ecem Altaş, Emine Akdemir, Gül Gönül Kayar and Mine Gökalp
Department of Analytical Development, Abdi İbrahim R&D Center, Turkey
Submission: December 7, 2019; Published: December 15, 2020
*Corresponding author: Ecem Altaş, Department of Analytical Development, Abdi İbrahim R&D Center, Istanbul, Turkey
How to cite this article:Ecem A, Emine A, Gül G K, Mine G. Alcohol-Induced Dose Dumping Studies in Gastro-Resistant Hard Gelatine Capsules Containing Immunosuppressant Drug. Glob J Pharmaceu Sci. 2020; 8(1): 555729. DOI: 10.19080/GJPPS.2020.08.555729.
Abstract
Introduction: The purpose of this study is to evaluate the risk of alcohol-induced dose dumping of gastro-resistant hard gelatin capsules containing immunosuppressant drug used to medicate relapsing types of multiple sclerosis (MS). Such studies are essential to evaluate the rate change of drug release from the dosage form, if the patient took alcoholic beverages in daily life. According to the EMA guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms, every altered release dosage form has to be evaluated for the hazard from alcohol induced dose dumping and / or changes in dissolution profiles (EMA/CHMP/QWP, 2014). Concerning these requirements, no differentiation is made between prolonged / sustained release products which normally contain larger doses (often double or more) as conventional immediate release (IR) forms and enteric coated preparations which should behave like IR products after dissolution of the functional coating and, thus, do not contain larger doses. Consequently, “instantaneous” release of the entire dose should not generate a risk for the patient. Nonetheless, impact of ethanol on drug release should also be investigated with these products. Results obtained from such tests should be carefully interpreted with respect to their therapeutic relevance.
Experimental: In case of generic product development (Tenipra 240 mg Gastro-resistant Hard Capsules®), these studies need to be done in comparison to reference product (Tecfidera 240 mg Gastro-resistant Hard Capsules®). This comparison is crucial since some modified-release oral dosage forms include active substances and/or excipients that shows higher solubility in ethanolic solutions compared to water and other buffer media. Hence, in vitro studies of drug release in alcoholic solutions of gastro-resistant hard capsules including immunosuppressant drug have been carried out. Bootstrap method has been used to calculate the similarity between 0.1 N HCl medium and 0.1 N HCl + 5 % ethanol, 0.1 N HCl + 10 % ethanol and 0.1 N HCl + 20 % ethanol media.
Result: Based on the obtained results, it was demonstrated that alcohol sensitivity is slightly more pronounced in case of the reference product compared to the test product.
Keywords:Alcohol induced-dose dumping; Modified release; Multiple sclerosis; Gastro-resistant hard gelatine capsules; Bootstrap
Introduction
Mankind has been dealing with multiple sclerosis disease since the dawn of human race. The first definition of multiple sclerosis (MS) dates back to the 14th century [1]. Furthermore, MS International Federation states that the disease tends to be growing in number amongst the people and today more than 2.8 million people has been diagnosed with MS in the world [2]. MS is an autoimmune illness that directly affects the nervous system [3] by attacking myelin and axons of the nerves [4]. There have been four major types of MS defined as relapsing remitting MS (RRMS), primary progressive MS (PPMS), secondary progressive MS (SPMS), and progressive relapsing MS (PRMS) [5]. The active substance, Dimethyl Fumarate, is thought to work by activating a protein called ‘Nrf2’ that regulates certain ‘antioxidant’ genes involved in protecting cells from damage of various form of multiple sclerosis [6]. Dimethyl fumarate is a white crystalline powder, nonhygroscopic [7,8] BCS class 1 (highly soluble and highly permeable) [7,8]. The structural formula of the Dimethyl Fumarate has been given in Figure 1 [9]. Dimethyl fumarate is used as an orally treatment option for RRMS with improved gastroenteric coated formulation [10]. DMF is metabolized to monomethyl fumarate (MMF) in the small intestine [11].
The mode of action comprises immunomodulatory effects and an activation of nuclear (erythroid derived 2) related factor mediated antioxidative response pathways leading to additional cytoprotective effects. Dimethyl Fumarate, 240 mg twice daily, has reduced relapse rates by approximately 50 % compared to placebo in two pivotal phase III trials. The studies revealed a beneficial safety profile of the molecule [12]. The drug, marketed as Tecfidera 240 mg Gastro-resistant Hard Capsules® contains dimethyl fumarate and has been indicated for MS has received approval by the US Food and Drug Administration (FDA) in 2013 [13]. In the European Union, the medication has received approval by the European Medicines Agency (EMA) in 2014 [14]. The appearance of the Tecfidera 240 mg Gastro-resistant Hard Capsules® is white to off white enteric coated mini tablets filled in size ‘0’ hard gelatin capsule with light green opaque body printed with “(240 mg) ^(BG-12) “ in black ink and opaque light green containing mini tablets. Microcrystalline cellulose, croscarmellose sodium, talc, silica, colloidal anhydrous, magnesium stearate, triethyl citrate, methacrylic acid – methyl methacrylate copolymer (1:1), methacrylic acid – ethyl acrylate copolymer (1:1) dispersion 30 %, simeticone, sodium laurilsulfate, polysorbate 80 are used for the capsule content (enteric-coated microtablets). Gelatin titanium dioxide (E171), brilliant blue FCF (E133) and yellow iron oxide (E172) are used for the capsule shell [15]. Tenipra 240 mg Gastro-resistant Hard Capsules® were developed and produced by Abdi İbrahim R&D Center (Istanbul, Turkey) with similar technology to Tecfidera 240 mg Gastro-resistant Hard Capsules®. The product development has aimed at developing a generic product that is safe, efficacious and bio equivalent to the reference product. The scientific approaches for pharmaceutical development studies have been based on the properties of the drug substance, the characterization of the reference product label claim and the intended patient population. The experiments have been designed in such a way to establish the concentration of each excipients with a step wise focus on parameters such as disintegration and dissolution. Excipients have been optimized to ensure minimum required levels were used to make a stable and safe product. The excipients selected are widely used in oral pharmaceutical formulations and they are sourced from approved manufacturers/suppliers. The quantity of excipients used in the final formulation is within safety margins and within the usual range generally used in oral pharmaceutical solid preparations. The selection of excipients for the formulation development has been based on the physicochemical properties of drug substance and compatibility with the excipients, which are also used in the approved UK reference product named Tecfidera 240 mg Gastroresistant Hard Capsules® and marketing authorization holder Biogen Idec Ltd. Manufacturing steps are dispensing & sifting, blending, lubrication, compression, seal coating, enteric coating, capsule filling and packing respectively. Dimethyl Fumarate mini tablets consist drug along with diluent, flow enhance & disintegrant agent. Core tablets were coated with first seal coating (Eudragit L 100) & followed by Enteric coat (Eudragit L 30 D55). 50 enteric coated mini tablets were filled in size “0” empty hard gelatin capsules. The product was developed keeping in mind its intended clinical use, bio availability, dosage form, strength, route of administration, safety, and efficacy of the product.
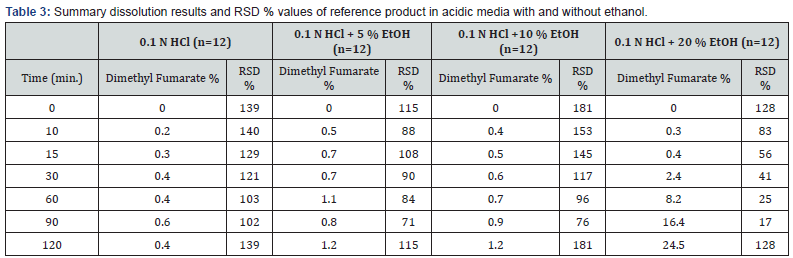
The objective of the product was to develop a robust, stable of delayed release capsule included the molecule named Dimethyl Fumarate pharmaceutically equivalent to the reference product. Initial development study was conducted based on the delayed release mini tablets in capsule against the reference product. In this study, in vitro studies were carried out to evaluate the risk of alcohol-induced dose dumping of Tecfidera 240 mg Gastro-resistant Hard Capsules® and Tenipra 240 mg Gastroresistant Hard Capsules® containing immunosuppressant drugs used to treat recurrent multiple sclerosis (MS) types by taking into recommendations of EMA guideline dose dumping is the undesired, rapid release of all or a significant part of the drug in a modified-release (MR) dosage form in a short period of time. The EMA guideline recommends performing in vitro studies of release in alcohol solutions, as some modified release oral dosage forms contain active substances and / or excipients that exhibit higher solubility in ethanolic solutions compared to water, as consumption of alcoholic beverages with such products may cause dose dumping. The guideline further mentions that “depending on the therapeutic indication and the therapeutic index of an active substance, dose dumping can pose a significant risk to the patients, either due to safety issues or diminished efficacy or both” (EMA, 2013). In case of enteric coated dosage forms the main concern may be related to the release of a relevant amount of an acid labile API already in the stomach. These once-daily capsules released 80 % of their substances in the first 15 minutes in an environment that has 20 % alcohol. This implies that if the patient takes the capsule with a high-alcohol drink such as vodka, liquor or [16]. In order to investigate the interaction with alcohol, experiments have been conducted by adding 5 %, 10 % and 20 % ethanol to selected acidic medium and the results were compared with obtained without ethanol presence in dissolution media for both test and reference product of Gastro-resistant Hard Gelatine Capsules by considering the EMA guide suggestions [17]. Since non-significant dissolved amount results have been obtained and RSD % values have been found more than 10 % for acidic media, f2 statistics cannot be used as outlined in Guideline on The Investigation of Bioequivalence CPMP/EWP/QWP/1401/98: ‘’The relative standard deviation or coefficient of variation of any product should be less than 20 % for the first point and less than 10 % from second to last time point’’.
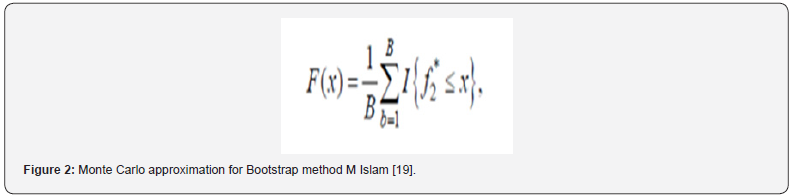
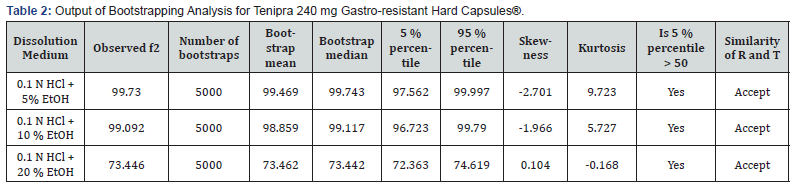
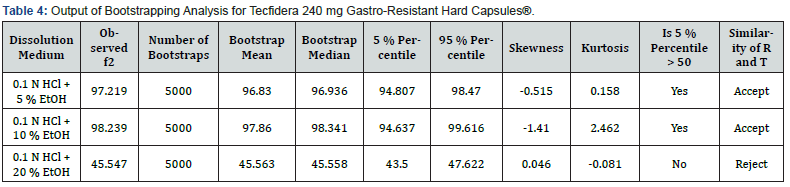
However, regardless of whether the conditions to adequately apply f2 in a dissolution experiment are fulfilled or not, the properties of the f2 sampling distribution do not allow the derivation of exact confidence intervals to adequately quantify the uncertainty of the f2 estimate. To address this, bootstrap methodology could be used to derive confidence intervals for f2 based on quantiles of resampling distributions, and this approach could actually be considered the preferred method over f2 and MD (‘EMA’s current practice and challenges in the evaluation of dissolution profile comparisons in support of minor/moderate product quality changes – Case Studies’, 2019). Bootstrap f2 is used as a confidence interval as well and the acceptance of similarity is considered if the lower bound of the non-parametric 90 % confidence interval is not less than 50 [18]. Bootstrapping is a computer-intensive approach to statistical inference. It is based on the sampling distribution of a statistic obtained by resampling from the data with replacement. When it is hard to derive the exact sampling distribution of certain statistics and their characteristics, bootstrap methods are used to approximate them. The characteristics include standard error, bias, skewness, critical values, mean squared error, and others. To derive an exact sampling distribution of a statistic of interest, the underlying population distribution from which sample is drawn has to be known. Two types of bootstrap methods are used that parametric and non-parametric to determine the sampling distribution of the statistic f ̂2 and its characteristics. Using both techniques 90 % confidence intervals for f2 is constructed. The distribution of f ̂2 can be estimated by using the bootstrap with the Monte Carlo approximation equation that is given in Figure 2. f2* is the value of the f2 based on both bootstrap sample and F(x)s a bootstrap estimator of the distribution function of f ̂2 based on the data X1, X2, Xn. The expected value, variance, skewness, kurtosis, and bias of the bootstrap sampling distribution of f ̂2 are estimated from F(x) [19]. Dissolver is a free software program which can perform most techniques for comparing drug release data, including the bootstrap f2 method [20]. L. Nocea L Gwaza, V. Mangas-Sanjuan and A. Garcia-Arieta state that in their articles when three different similarity factors, f2, E(f2) and bc-f2, and their corresponding 90 % confidence intervals were calculated by using three free software programs as Pheq bootstrap, Bootf2bca and DD Solver, all three platforms have been reported the same value [21]. Therefore, similarity between 0.1 N HCl medium and 0.1 N HCl + 5 % Ethanol, 0.1 N HCl + 10 % Ethanol and 0.1 N HCl + 20 % Ethanol media was calculated with Bootstrap by using DD solver software.
Experimental
Chemicals and reagents
The chemicals that are used in the study were of analytical reagent grade. Acetonitrile (ACN), perchloric acid (HClO4), hydrochloric acid (HCl), methanol (MeOH), ethanol (EtOH), potassium dihydrogen phosphate (KH2PO4), sodium hydroxide (NaOH) and phosphoric acid (H3PO4) were purchased from Merck (Darmstadt, Germany) and JT Baker (Center Valley, USA). Deionized and distilled water was used from PURELAB® Ultra Elga DV35 (High Wycombe, United Kingdom). Dimethyl Fumarate standard was obtained from Enaltec Labs Pvt. Ltd. (India). It was used enteric coated tablets produced by Abdi İbrahim, Research and Development Center Department (Istanbul, Turkey).
Mobile phase
The mobile phase was prepared by 2 ml perchloric acid in 1000 ml of water and mixed. It was filtered through a 0.45 μm membrane filter (Millipore, MA, USA) and degassed by sonication [22-26].
Chromatographic equipment and conditions
ACQUITY H-Class System (Waters, Milford, USA) we used to consist of a Quaternary Solvent Manager (QSM), a Sample Manager with Flow Through Needle (SM-FTN) design and UV&PDA detector. UV&PDA deduction was carried out with set to 210 nm. The system consisted of the data that were collected and evaluated by using Waters Empower 2 software. GL Science Inertsil ODS-3 150 mm x 4.6 mm with 5μm column was used in the method for the determination of dissolution rate for Dimethyl Fumarate. The column temperature was set as 35 ºC.
Standard preparations
Stock solution of the Dimethyl Fumarate standard (0.96 mg mL 1) was prepared by dissolving with the relevant medium (0.1 N HCl, 0.1 N HCl + 5 % EtOH, 0.1 N HCl + 10 % EtOH, 0.1 N HCl + 20 % EtOH) in which the study will be performed. Standard solutions were prepared from this stock solution that contains 0.24 mg mL-1 by dissolving with the relevant medium.
Sample preparations
Tenipra 240 mg Gastro-resistant Hard Capsules® samples were produced by Abdi İbrahim İlaç Sanayi Tic. A.Ş. (Istanbul, Turkey) and Tecfidera 240 mg Gastro-resistant Hard Capsules® were supplied from Biogen Idec GmbH (Germany) are used as sample. Placed 500 ml relevant dissolution medium into each 6 vessels and warmed up to 37 ºC ± 0.5 ºC. Throwed one capsule into each vessel and start the analysis at 100 rpm speed with paddle (USP II). At the end of 120 minutes taken sample solution from each vessel into vials.
Results and Discussion
Comparative Dissolution Results For The Test Product (Tenipra 240 mg Gastro-resistant Hard Capsules®)
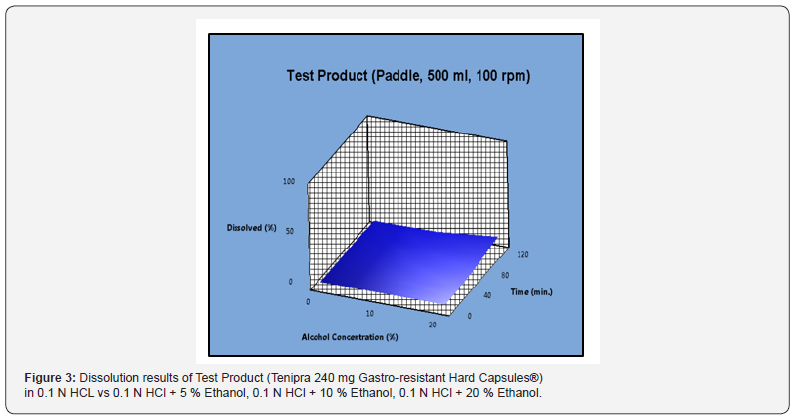
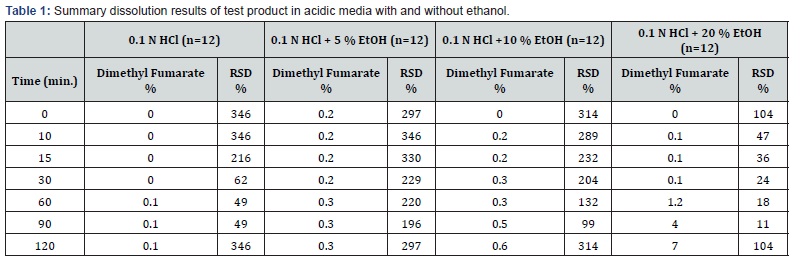
Dissolution results and RSD % values of Tenipra 240 mg Gastro-resistant Hard Capsules® for 0.1 N HCl, 0.1 N HCl + 5 % Ethanol, 0.1 N HCl + 10 % Ethanol and 0.1 N HCl + 20 % Ethanol media have been reported in Table 1. Dissolution results of the test product obtained in 0.1 N HCl medium have been compared with those obtained in 0.1 N HCl + 5 % Ethanol, 0.1 N HCl + 10 % Ethanol and 0.1 N HCl + 20 % Ethanol media to evaluate the risk of alcohol dose dumping. It was observed that dissolution results in all media were below than 10 %, RSD % values have been found more than 10 % and there is no significant change according to without ethanol results. According to EMA guideline, similarity was calculated with bootstrapping method and the result has been shown in Table 2. It has been proved by using bootstrap method that the solubility curves in 0.1 N HCl medium and 0.1 N HCl + 5 % ethanol, 0.1 N HCl + 10 % ethanol, 0.1 N HCl+ 20 % ethanol media are similar. Dissolution results of the test product (Tenipra 240 mg Gastro-resistant Hard Capsules®) in 0.1 N HCL vs 0.1 N HCl + 5 % ethanol, 0.1 N HCl + 10 % ethanol, 0.1 N HCl + 20 % ethanol has been shown in Figure 3.
Comparative Dissolution Results For The Reference Product (Tecfidera 240mg Gastro-Resistant Hard Capsules®)
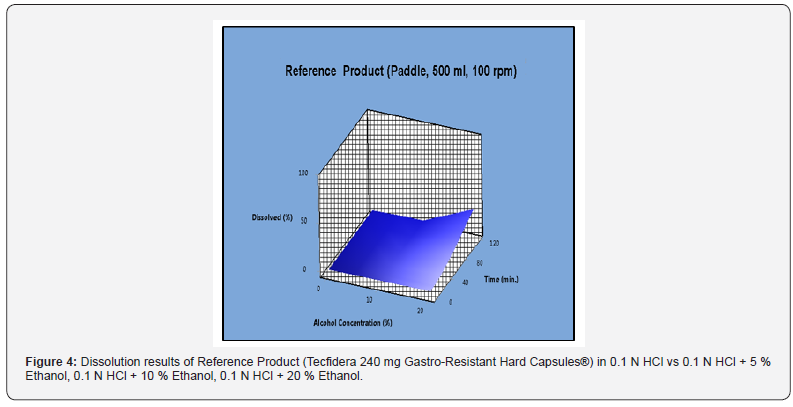
Dissolution results and RSD % values of Tecfidera 240 mg Gastro-resistant Hard Capsules® for 0.1 N HCl, 0.1 N HCl + 5 % Ethanol, 0.1 N HCl + 10 % Ethanol and 0.1 N HCl + 20 % Ethanol medium are reported in Table 3. Dissolution results of reference product obtained in 0.1 N HCl medium have been compared with those obtained in 0.1 N HCl + 5 % ethanol, 0.1 N HCl + 10 % ethanol and 0.1 N HCl + 20 % ethanol media to evaluate the risk of alcohol dose dumping. It was observed that although dissolution results in 0.1 N HCl + 5 % ethanol and 0.1 N HCl + 10 % ethanol media were below than 10 %, dissolution results of 0.1 N HCl + 20 % ethanol medium has got significant change for the reference product. It also was stated in the evaluation report of Tecfidera 240 mg Gastro-resistant Hard Capsules® published by FDA that alcohol dumping effect was observed existing 20 % EtOH media (FDA, 2009). RSD % values have been found more than 10 % for all dissolution media. According to EMA guideline, similarity was calculated with bootstrapping method and the result has been shown in Table 4. It has been proved by using bootstrap method that the solubility curves of 0.1 N HCl medium and 0.1 N HCl + 5 % ethanol, 0.1 N HCl + 10 % ethanol media are similar. However, the solubility curves of 0.1 N HCl and 0.1 N HCl + 20 % ethanol is not similar for the reference product. Dissolution results of the reference product (Tecfidera 240 mg Gastro-Resistant Hard Capsules®) in 0.1 N HCl vs 0.1 N HCl + 5 % ethanol, 0.1 N HCl + 10 % ethanol, 0.1 N HCl + 20 % ethanol has been shown in Figure 4.
Conclusion
According to the results of the test product named Tenipra 240 mg Gastro-resistant Hard Capsules®, no significant change has been observed for dissolution profile in the presence of low or high concentration of ethanol and it has been proved by using bootstrap method that the solubility curves in 0.1 N HCl medium and 0.1 N HCl + 5 % ethanol, 0.1 N HCl + 10 % ethanol, 0.1 N HCl+ 20 % ethanol media are similar. The dissolution test for reference product (Tecfidera 240 mg Gastro-Resistant Hard Capsules®) in the presence of alcohol was done for informatory purpose. According to the results, significant increase has been observed in dissolution values in the presence of 20 % alcohol as stated also in the FDA evaluation report of Tecfidera 240 mg Gastro-Resistant Hard Capsules® (FDA, 2009). Bootstrap method has been used to calculate the similarity factor between 0.1 N HCl medium and 0.1 N HCl + 5 % ethanol, 0.1 N HCl + 10 % ethanol and 0.1 N HCl + 20 % ethanol media. Based on the obtained results, it was demonstrated that alcohol sensitivity is slightly more pronounced in case of the reference product compared to the test product named Tenipra 240 mg Gastro-resistant Hard Capsules®. Overall, results do not support dose dumping risk from test product. In summary, it has been proved that the test product with similar production technology to the reference product shows safer dissolution profile with increasing alcohol percentage (especially at 20 % ethanol level), and there is no risk of alcohol dose dumping.
References
- Kumar DR (2011) Jean-martin charcot: The father of neurology. Clinical Medicine and Research 9(1): 46-49.
- (2020) MS International Federation. What is MS.
- Dobson R, Giovannoni G (2019) Multiple sclerosis– a review. European Journal of Neurology 26(1): 27-40.
- Thomas FP (2012) Multiple Sclerosis, Pathy’s Principles and Practice of Geriatric Medicine: (5th), 1(3): 823-833.
- Ghasemi N, Razavi S, Nikzad E (2017) Multiple Sclerosis: Pathogenesis, Symptoms. Diagnoses and Cell Based Therapy.
- Holm Hansen R (2020) Dimethyl fumarate therapy reduces memory T cells and the CNS migration potential in patients with multiple sclerosis. Multiple Sclerosis and Related Disorders 37: 101451.
- Ojha S, Kumar B (2016) Formulation and optimization of chitosan nanoparticles of dimethyl fumarate using box-behnken design. International Journal of Applied Pharmaceutics 8(4): 10-17.
- Ojha S, Kumar B (2018) Preparation and statistical modeling of solid lipid nanoparticles of dimethyl fumarate for better management of multiple sclerosis. Advanced Pharmaceutical Bulletin 8(2): 225-233.
- KooijmanH(2004) Dimethyl fumarate. ActaCrystallographica Section E: Structure Reports Online 60(5): 917-918.
- Suneetha A, Raja Rajeswari K (2016) Role of dimethyl fumarate in oxidative stress of multiple sclerosis: A review. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences 1019: 15–20.
- Yadav SK (2019) Insight into the mechanism of action of dimethyl fumarate in multiple sclerosis. Journal of Molecular Medicine 97(4): 463-472.
- Linker RA, Gold R (2013) Dimethyl fumarate for treatment of multiple sclerosis: Mechanism of action, effectiveness, and side effects. Current Neurology and Neuroscience Reports 13(11).
- FDA (2013) Tecfidera Product Label. Food and Drug Administration, Pp. 1-15.
- Toumi M, Jadot G (2014) Economic impact of new active substance status on EU payers’ budgets: example of dimethyl fumarate (Tecfidera ®) for multiple sclerosis. Journal of Market Access & Health Policy 2(1): 23932.
- Michailidou A, Trenz HJ, de Wilde P (2019) Annex I, The Internet and European Integration167-172.
- European Medicines Agency (EMA) (2010) European Medicines Agency concludes review of modified release oral opioids of the WHO level III scale for the management of pain 44: 23-25.
- Fava S (2020) Alcohol-induced dose dumping:A comparative study of generic and innovator product13(1): 1-2.
- Paixão P (2017) Evaluation of dissolution profile similarity-Comparison between the f2, the multivariate statistical distance and the f2 bootstrapping methods. European Journal of Pharmaceutics and Biopharmaceutics 112: 67-74.
- M Islam, M. (2018) Bootstrap confidence intervals for dissolution similarity factor f2. Biometrics & Biostatistics International Journal 7(5): 397-403.
- Zhang Y (2010) DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS Journal 12(3): 263-271.
- Noce L (2020) Comparison of free software platforms for the calculation of the 90% confidence interval of f2 similarity factor by bootstrap analysis. European Journal of Pharmaceutical Sciences 146: 105259.
- (2019) EMA’s current practice and challenges in the evaluation of dissolution profile comparisons in support of minor/moderate product quality changes-Case Studies.
- EMA/CHMP/QWP (2014) Guideline on quality of oral modified release products. European Medicines Agency 44: 1–16.
- EMA (2013) Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms (EMA / CPMP / EWP / 280 / 96 Corr1. European Medicines Agency(EMEA) 44: 1-38.
- FDA (2009) Center for Drug Evaluation and Clinical Pharmacology and Biopharmaceutics Review S. Food and Drug Administration, pp. 1-5.
- Ghasemi N, RazaviSh, Nikzad E (2020) Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell Journal 19(191): 1-10.






























