Formulation and Evaluation of Liquid Nanocrystals of Sorafenib Tosylate
Nagaraju Diddi*, Shravan Kumar Y, Pavani S and Neelima P
Department of Pharmaceutics, Vaagdevi College of Pharmacy, India
Submission: August 18, 2019; Published: October 03, 2019
*Corresponding author: Nagaraju Diddi, Department of Pharmaceutics, Vaagdevi College of Pharmacy, India
How to cite this article: Nagaraju D, Shravan K Y, Pavani S, Neelima P. Formulation and Evaluation of Liquid Nanocrystals of Sorafenib Tosylate. Glob J Pharmaceu Sci. 2019; 7(5): 555721. DOI: 10.19080/GJPPS.2019.07.555721.
Abstract
The liquid crystalline state has both the properties of liquid and solid. The liquid state is found to associate with flow property whereas the solid state has structural properties of crystallinity in aspects of orientation and position. Liquid crystalline phases represent intermediate states and are also called as mesophases.
The studies were done with different formulations to ensure its controlled drug release and bioavailability.
Context: Nanoparticles helps in site specific targeting which aids in controlled release of the incorporated drugs. Site specific targeting can be achieved by attaching targeting ligands to surface of particles with the help of magnetic field influence.
Aim and objective: To ensure that nanoparticles will provide control release of the drug which are used as drug carriers for lipophilic molecules there by which it enhances the solubility and bioavailability of poorly water-soluble drug by reducing their doses regimen as a drug delivery system.
Methods and material: Simple emulsification followed by high pressure homogenisation
Results: FTIR Studies, Characterization of particle size, entrapment efficiency and total drug content.
Conclusions: Liquid crystal nanoparticles provide controlled release of the drug and these systems are used as drug carriers for lipophilic drugs, to enhance the solubility and bioavailability of poorly water-soluble drugs and to reduce the doses regimen through nanoparticles, as a drug delivery system.
Keywords: Nanocrystals; Targeted Delivery; Bioavailability; High Pressure Homogenization; Scanning Electron Microscope; Ultrasonication; Poloxamer 407 And Glycerol Mono Oleate
Introduction
Nanoparticles are defined as particulate dispersion or solid particles with size in range of 10-1000 nm. Polymeric nanoparticles are made from non-biodegradable and bio-degradable polymers. Sorafenib is a potent anticancer agent for the treatment of hepatocellular carcinoma. It exists in crystalline as well as amorphous form, of these later exhibits higher bioavailability owing to improved solubility [1-3]. Solubility can be improved by mechanisms like co-solvency, complexation, chemical modification, hydrotropy, size reduction and changing crystal morphism etc. To compare the surface area of different materials quantitatively, the term specific surface area is used. This is defined as the surface area per unit weight or volume of material. As surface area increases, solubility increases [4].
Methodology
Pre-formulation studies
The goals of pre-formulation studies were meant to select a suitable drug substance, evaluate its physical and chemical properties and understand the material’s stability under the conditions that will enforce the development of a drug delivery system. It Establishes compatibility with excipients and enhances physico-chemical properties and determines its kinetic release rate profile [5-9].
Formulation and development
Materials
Sorafenib tosylate, Glycerol mono oleate, and Poloxamer 407.
Preparation of standard curve
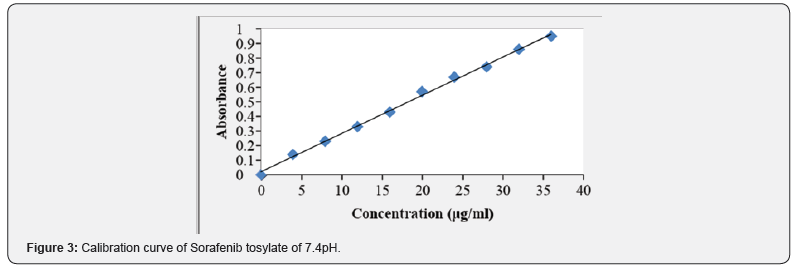
10mg of Sorafenib tosylate was dissolved 10mL of 7.4 pH buffer (stock solution) 1mL solution was taken and make up with 10mL of 7.4 pH (10μg/mL) containing 2, 4, 6, 8, 10μg/ mL of sorafenib per mL of solution [10-12]. The absorbance of the above dilutions was measured at 264 nm by using UVSpectrophotometer taking 7.4 pH buffer as blank. Then a graph was plotted by taking concentration on X-Axis and absorbance on Y-Axis which gives a straight-line Linearity of standard curve which was assessed from the square of correlation coefficient (R2) which determined by least-square linear regression analysis [13].
Preparation of sorafenib liquid crystal nanopaticles
Formulations containing 10mg Sorafenib were prepared by emulsification technique to study the effect of method of manufacture on drug release with the help of High-pressure homogenization. High pressure homogenization (HPH) is a reliable and powerful technique for the preparation of liquid crystal nanoparticles [14-16]. High pressure homogenizers tend to push the liquid with high pressure (100-2000 bar) through a narrow gap (in the range of a few microns). The fluid accelerates on a very short distance to very high velocity (over 1000km/h). Very high shear stress and cavitation forces disrupt the particles down to the submicron range. Typical lipid contents are in the range 5-10% and represent no problem to the homogenizer [17].
Method of preparation
The Excipients were weighed and moltened separately in a china dish over a water bath. Drug was added to the molten polymer and mixed well [18]. After thorough mixing the china dish was removed from water bath and cooled. The coherent mass was then treated for high pressure homogenization [19- 22] (Table 1).
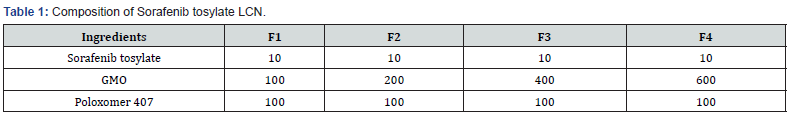
Evaluation of liquid crystal nanoparticles
Drug Content (Assay)
The standards and requirements were maintained accordingly and an accurately weighed portion of the powder equivalent to about 10mg of Sorafenib tosylate was transferred to a 10mL volumetric flask containing 7.4 pH. It was shaken by mechanical means for 1h then it was filtered through a Whattman filter paper (No.1) and diluted to 1mL with 7.4 pH. and absorbance was measured against blank at 264nm [23].
In vitro drug release studies
The In vitro drug release study was performed for all the tablets using Franz diffusion cell apparatus under the following conditions [24].
Dissolution test parameters
Medium: 25mL of 7.4 pH buffer
Rotation speed: 50rpm
Temperature: 37±0.5ºC
Sampling volume: 5 mL
Sampling time: 0.5, 1, 2, 3, 6, 9, 12 hours
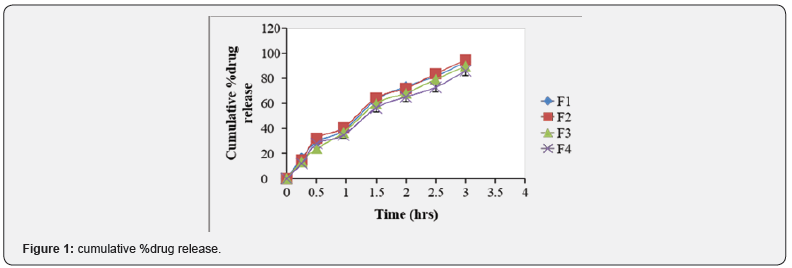
The samples withdrawn were filtered through Whatman filter paper (No.1) and drug content in each sample was analyzed by UV-visible spectrophotometer at λ max 264nm [25- 27] (Figure 1) (Table 2).

Results
FTIR Studies
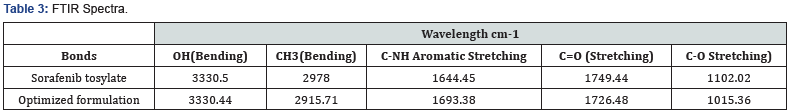
Compatibility of the Sorafenib tosylate with polymers was studied and FTIR spectral analysis was carried out to identify the changes in chemical composition of the drug after combining with the excipients. The pure drug mixture was mixed properly Placed under Bruker FTIR scanned in wavelength. The spectra were analyzed and interpreted [28] (Figure 2) (Tables 3 & 4).


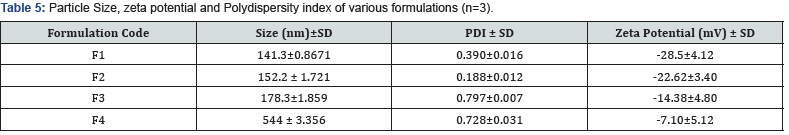
Characterization of prepared sorafenib liquid crystal nanoparticles
Particle Size, zeta potential and Polydispersity index of prepared LCNs: All the prepared samples were analyzed in order to determine their particle size distribution, zeta potential and PDI values [29] (Figure 3) (Table 5).
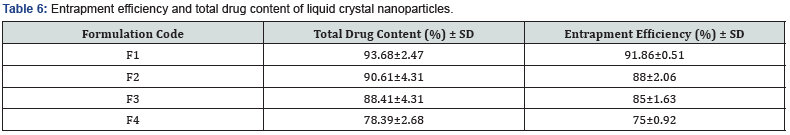
Determination of entrapment efficiency and total drug content
All the formulations were analyzed for entrapment efficiency (Table 6). Results are tabulated and represented [30] (Figure 4).
Discussion
Sorafenib tosylate liquid crystal nanoparticles were successfully prepared by high pressure homogenization followed by the ultrasonication method along with bath sonication by using ingredients such as glycerol mono oleate, poloxomer 407. Various formulation factors on particle size, zeta potential, polydispersity index, entrapment efficiency, drug content, in vitro and ex vivo studies were also studied Formulation F1 with poloxamer 407 with lipid and glycerol monooleate concentration 2:1 showed lower particle size of 142nm, zeta potential -34.1 mV, polydispersity index of 0.324 with uniform dispersion, along with the highest entrapment efficiency 91.86% and drug release of 90.03% [31].
Liquid crystal dispersion prepared using Poloxamer 407 (1.5%) as stabilizer [F4] showed lower particle size than the other surfactants irrespective of the lipids studied [32-34]. This result has been explained due to the higher molecular weight of Poloxamer 407 and higher HLB value of Poloxamer 407. Thus, it could be well concluded that the amount of drug released was much slower and controlled from the liquid crystal dispersions prepared by using glycerol monooleate as the lipid matrix, with poloxamer 07 as the surfactant, than that from the sorafenibpure drug solution From correlation coefficient (R2 ) value of different kinetic models, it was found that the formulation (F4) sorafenib –dispersion containing poloxamer 407 and glycerol monooleate in the ratio of 1:4 showed R2 of 0.9748 of which followed Higuchi model with ‘n’ value of 0.7382 indicating drug release followed non-fickian diffusion [35].
Conclusion
The sorafenib tosylate liquid crystal nanoparticles with the application of high-pressure homogenizer followed by ultrasonication method along with the bath sonication turned out to be a useful method for the successful incorporation of the poor water-soluble drug sorafenib with high entrapment efficiency. Furthermore, it could be presumed that if the nanometer range particles were obtained, the bioavailability might be increased. Hence, we can conclude that liquid crystal nanoparticles provide controlled release of the drug and these systems are used as drug carriers for lipophilic drugs, to enhance the solubility and bioavailability of poorly water-soluble drugs and to reduce the doses regimen through nanoparticles, as a drug delivery system.
References
- Alfonso R Gennaro (2000) The Science and Practice of Pharmacy (20th Edn). Philadelphia: Lippincot Williams and Wilkins. 1: 903.
- Anvesh Kumari, Sudesh Kumar Yadav, Subhash C Yadav (2010) Biodegradable Polymeric Nanoparticles-based Drug Delivery Systems. Colloids and Surfaces B: Biointerfaces 75(1): 1-18.
- B Siekmann, K Westesen (1992) Sub-Micron sized Parenteral carrier systems. Pharm. Pharmacol. Lett 1: 123-126.
- C Freitas C, Müller RH (1998) Effect of light and Temperature on zeta Potential and Physical stability in nanoparticles dispersions. Int J Pharm 168(2): 221-229.
- CM O'Driscoll, BT Griffin (2008) Biopharmaceutical Challenges Associated with Drugs with low aqueous solubility-the Potential Impact of Lipid-Based Formulations. Advanced Drug Delivery Reviews 60(6): 617-624.
- Cavalli R, Gasco MR, Chetoni P (2002) Solid lipid Nanoparticles (SLN) as ocular Delivery System for tobramycin. Int J Pharm 238(1-2): 241-245.
- Chunbai He, Yiping Hu, Lichen Yin (2010) Effects of Particle size and Surface Charge on cellular uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 31(13): 3657-3666.
- De Campos A, Sanchez A, Alonso MJ (2001) Chitosan Nanoparticles: a New Vehicle for the Improvement of the ocular Retention of drugs. Application to Cyclosporin A. Int J Pharm 224(1-2): 159-168.
- Decuzzi P, Godin B, Tanaka T (2010) Size and shape effects in the biodistribution of intravascularly injected particles. Journal of Controlled Release 141(3): 320-327.
- Donald E, Owens III, Nicholas A Pappas (2006) Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles, International Journal of Pharmaceutics 307(1): 93-102.
- Elias Fattal, Christine Vauhier (2002) Encyclopedia of Pharmaceutical Technology (2nd Edn), New York: Marcel Dekker, Inc., pp. 1864.
- Gilbert S Banker, Christopher T Rhodes (2002) “Colloidal Drug Delivery Systems” (4th Edn) Modern Pharmceutics 121: 167-172.
- Hazen Ali, Amit B Shirode, Paul W Sylvester, Sami Nazzal (2010) Preparation, Characterization and anticancer effects of simvastatin-tocotrienol lipid nanoparticles. International Journal of Pharmaceutics 389(1-2): 223-31.
- Herbert A Libermann, Martin M Rieger, Gilbert S Banker (2005) Pharmaceutical Dosage Forms. (2nd edn), Disperse Systems 3: 103-190.
- Indian Pharmacopoeia (2007) Govrnament of India Ministry of Health and Family Welfare. 1: 2 (2).
- J Prasad Raoa, Kurt E Geckeler (2011) Polymer nanoparticles: Preparation techniques and size-control parameters. Progress in Polymer Science 36(7): 887-913.
- Katy Margulis-Goshen, Shlomo Magdassi (2009) Formulation of simvastatin nanoparticles from microemulsion, Nanomedicine: Nanotechnology. Biology and medicine 5(3): 274-281.
- Khin Yin Win, Si-Shen Feng (2005) Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 26(15): 713-2722.
- Kumaresh S Soppimatha, Tejraj M Aminabhavia, Anandrao R Kulkarnia, Walter E Rudzinski (2001) Biodegradable polymeric nanoparticles as drug delivery devices. Journal of Controlled Release 70: 1-20.
- Leon Lachman, Herbert A Liberman, Joseph L Kanig (1991) The Theory and Practice of Industrial Pharmacy. Bombay: Varghese Publishing House, (3rd Edn). pp. 430-439.
- Maheshwari Manish, Jahagirdar harshal, Paradkar Anant (2005) Melt sonocrystallisation of ibuprofen: Effect on crystal properties. European journal of pharmaceutics and biopharmaceutics 25(1): 41-48.
- Melike Üner and Gülgün Yener (2007) Importance of Nanoparticlesin Various Administration routes and Future Perspectives. Int J Nanomedicine 2(3): 289-300.
- Mukesh S Patil, Kedar R Bavaskar, Ghanashyam A Girnar, Ashish S Jain, Avinash R Tekade (2001) Prepartion and optimization of simvastatin nanoparticle for solubility enhancement and in-vivo International Journal of Pharma Research and Development 2(12): 219-226.
- Müller RH, Mäder K, Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled Drug Delivery-a review of the state of the art. Eur J Pharm Biopharm 50: 161-177.
- Ponchel G, Montisci MJ, Dembri A (1997) Mucoadhesion of Colloidal Particulate Systems in the Gastro-Intestinal tract. Eur J Pharm Biopharm 44: 25-31.
- Ravindra S Dhumal, Shailesh V Biradar, Shigeo Yamamura, Anant R Paradkar, Peter York (2008) Preparation of amorphous cefuroxime Axetil nanoparticles by sonoprecipitation for enhancement of bioavailability. European journal of pharmaceutics and biopharmaceutics 70(1): 109-115.
- Runge S, Mehnert W, Müller RH (1996) Nanoparticles, A novel formulation for the oral administration of drugs. Eur J Pharm Sci 4: 132.
- Taizia D Silva, Jackson ALC Resende, Valquiria T Arantes, Nivaldo L Speziali, Renata B de Oliveira (2007) Solid dispersions of Simvastatin with polyethylene glycol to improve solubility, Latin-American Symposium on Polymorphism and Crystallization in Drugs and medicinces. 12: 1068.
- Venkateshwarlu V, Manjunath K (2004) Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J Control Rel 95(3): 627-38.
- Vikram M Pandya, Jayavadan K Patel, Dhaval J Patel (2011) Formulation and Optimization of Nanosuspensions for Enhancing Simvastatin Dissolution using Central Composite Design, Dissolution Technologies. pp. 40-45.
- Wolfgang Mehnert, Karsten Mader (2001) Nanoparticles Production, characterization and applications. Advanced Drug Delivery Reviews 47: 165-196.
- Yanzhuo Zhang, Jinghai Zhang, Tongying Jiang, Siling Wang (2011) Inclusion of the Poorly Water-Soluble Drug Simvastatin in Mesocellular Foam Nanoparticles: Drug Loading and Release Properties. International Journal of Pharmaceutics 410(1-2): 118-124.
- Zhiwen Zhang, Huihui Bu, Zhiwei Gao, Yan Huang, Fang Gao, Yaping Li (2010) The Characteristics and Mechanism of Simvastatin Loaded Nanoparticles to Increase oral Bioavailability in Rats. International Journal of Pharmaceutics 394(1-2): 147-153.
- Zimmermann E, Müller RH (2001) Electrolyte- and pH-stabilities of aqueous nanoparticle dispersions in artificial gastrointestinal media. Eur J Pharm Biopharm 52(2): 203-210.
- Zur Muhlen, C Schwarz, W Mehnert (1998) Solid lipid nanoparticles (SLN) for controlled drug delivery - drug release and release mechanism. Eur. J. Pharm. Biopharm 45(2): 149-155.






























