Carphedon at the Crossroads: A Dangerous Drug or a Promising Psychopharmaceutical?
Jiri Patocka1,2*
1Faculty of Health and Social Studies, University of South Bohemia České Budějovice, Czech Republic
2Biomedical Research Centre, University Hospital, Czech Republic
Submission:March 18, 2019; Published:June 04, 2019
*Corresponding author:Jiri Patocka, University of South Bohemia České Budějovice, Faculty of Health and Social Studies, Institute of Radiology, Toxicology and Civil Protection, České Budějovice, Biomedical Research Centre, University Hospital, Hradec Kralove, Czech Republic
How to cite this article: Jiri Patocka. Carphedon at the Crossroads: A Dangerous Drug or a Promising Psychopharmaceutical?. Glob J Pharmaceu Sci. 2019; 7(3): 555713. DOI: 10.19080/GJPPS.2019.06.555713.
Abstract
Carphedon is a phenyl derivative of the nootropic drug piracetam (Nootropil) and is effective in increasing physical endurance and cold resistance and is used for amnesia treatment. It was developed in Russia as a stimulant to keep astronauts awake on long missions, and occasionally used in Russia as a nootropic prescription for various types of neurological disease. It became well known a couple years ago when a leading nootropic supplier in California started selling it on the Internet as a supplement and a bunch of athletes got kicked out of the Olympics for illegal using it. Carphedon was found to activate the operant behavior more powerfully, to remove psychodepressant effects of diazepam, to inhibit post-rotational nystagmus, and to prevent the development of retrograde amnesia. Unlike piracetam, carphedon exhibits a specific anticonvulsant action. When given in high doses, produces psychodepressant effects. It is also claimed to increase physical stamina and provide improved tolerance to cold. As a result, it appears on the lists of banned substances issued by the World Anti-Doping Agency.
Keywords: Carphedon; Phenotropil; 2-(4-Phenyl-2-Oxopyrrolidin-1-Yl)Acetamide; Nootropic; Stimulant
Introduction
Carphedon (carphedone, fonturacetam, phenotropyl, 4-phenylpiracetam) was synthesized in 1990 by Russian chemists as a combination of two drugs - nootropic piracetam and amphetamine stimulant. It was developed as a medicine that improves the physical and psychological ability of astronauts to work at different stages of space travel. It was assumed that it could combine the properties of both groups of drugs not only in chemical but also in pharmacological terms. That is, it will have a beneficial effect on the psyche while improving cognitive brain function. An ideal combination of medicine that would certainly find its place in the military, astronautics and wherever a person is exposed to great physical and/or mental stress. Indeed, carphedon has been shown to increase physical performance, resistance to cold, and is useful as a drug in amnesia. The usefulness of carphedon as a medicine that helps astronauts to cope with problems in a weightless state has also aroused the interest of experts in civil medicine. In 2005, carphedon was launched on the Russian market as a new drug called Phenotropil [1,2].
Chemistry
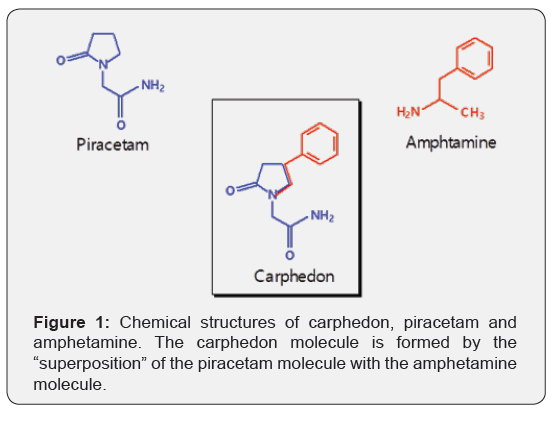
Carphedon, 2-oxo-4-phenyl-1-pyrrrolidine acetamide, C12H14N2O2, mol. wt. 218.25, CAS Number 77472-70-9, is a liquid compound having a density of 1.22 g / cm3 with a refractive index of 1.579 and boiling point 486.4° C at 760 mm Hg. Carphedon is a phenyl-derivative of piracetam (Nootropil) and also an amphetamine derivative. Part of its structure is identical to piracetam, part with amphetamine (Figure 1). Carphedone is a chiral compound existing in two stereoisomers, R and S. In medical practice it is used as a racemate.
Pharmacology
Carphedone can be considered pharmacologically nootropic, stimulant and adaptogen. As a substance with the nootropic effect of piracetam, it opens up new possibilities in the treatment of CNS diseases [3]. Phenotropil contains racemic carphedone, but its stereoisomers do not have completely identical pharmacological properties [4].
Pharmacokinetics
Carphedon is rapidly absorbed from the GIT and rapidly distributed to tissues. Its bioavailability in humans after p.o. administration is 100%. Maximum blood concentration is reached after 1 hour, half-life is 3 to 5 hours. It easily crosses the blood-brain barrier [3,5]. Carphedon is not metabolised in the body and is excreted unchanged - 40% in urine and 60% in bile and sweat [6]. In urine, carphedone can be quantitated by capillary gas chromatography with NP-detector [7] or mass spectrometer [8], so its abuse as doping can be easily controlled [9] .
Pharmacodynamics
Carphedon is primarily a nootropic drug [10]. It activates brain integration activities, improves memory, facilitates learning, increases the speed of information transfer between hemispheres of the brain, increases concentration on mental work, and increases resistance to brain hypoxia and poisons [11]. It has antiamnestic and anticonvulsant effects, acts anxiolytically, and induces good mood [3,5,12]. It also acts as an anorectic, beneficially affects brain metabolic processes and improves blood circulation in the ischemic areas of the brain [1] and enhances the body’s energy potential by improving glucose utilization [3]. In the rat model, carfedone at 100mg / kg (i.p.) was found to suppress the effect of scopolamine on cholinergic receptors, affect the density changes of dopaminergic and benzodiazepine receptors, and abolish its amnestic effect [13].
The stimulating effect of carphedon is evident in improving motor skills, increasing physical capacity for work and resistance to stress and fatigue [14]. The psychostimulant effect is to improve concentration on mental work and to improve mood [12]. Carphedone also has a slightly analgesic effect and increases the pain threshold [6].
The adaptogenic effect of carphedone results in increased resistance to stress under conditions of excessive mental and physical activity, fatigue, hypokinesia and low temperature immobilization. Peoples used carphedon claim that they have increased visual acuity and expanded their field of vision [3]. Carphedon has no effect on respiration or cardiovascular system. It stimulates the production of antibodies in response to antigen, increases immunity, but does not alter the skin’s allergic inflammatory response to foreign proteins. Carhedon does not induce dependence, tolerance and withdrawal syndrome [15].
Stereoselective Pharmacodynamics
All previously published works on carphedon were performed with the racemate of this drug. It has only recently been published [16] that the differences exist in the biological effects of both stereoisomers. The aim of the study was to compare stereoselective pharmacological activities of the R- and S-enantiomers of carphedon in various behavioral tests. Racemic carphedone served as a control. The amount of drug in the brain was measured by high performance liquid chromatography and tandem mass spectrometry (HPLC / MS / MS).
Significant increases in spontaneous motor activity (openfield test) were observed following single administration of R-carphedon at 10 and 50mg / kg and S-carphedon at 50mg / kg. In the forced swimming test, the R-carphedon showed an antidepressant effect at doses of 50 and 100mg / kg and S-carphedone only at a higher dose, i.e. 100mg / kg. R-carphedon had a significantly better effect in the passive avoidance test already at 1mg / kg, whereas S-carphedon was ineffective in this test up to 100mg / kg. Behavioral tests have shown that both carphedon enantiomers have an antidepressant effect and an ability to increase motor activity, but only R-carphedon improves memory, although the brain concentration of both enantiomers was the same. These results may be important for the clinical use of optically pure carphedone isomers [4].
Recently, it was shown that S-carphedon is a selective dopamine transporter (DAT) inhibitor that does not influence norepinephrine (NE) or serotonin (5-HT) receptors. S-Carphedon reduces the plasma glucose and leptin concentration and decreases hyperglycemia in a glucose tolerance test in both the mice and the rats. S-Carphedon did not influence locomotor activity in the obese Zucker rats or in the WD-fed mice. The results demonstrate that this compound reduces body weight gain and improves adaptation to hyperglycemia without stimulating locomotor activity. S-Carphedon could be potentially useful for treating obesity in patients with metabolic syndrome with fewer adverse health consequences compared to other anorectic agents [16].
Toxicology
The acute toxicity of carphedon is low, the median lethal dose (LD50) for mouse at p.o. administration is 1100mg / kg [17], a lethal dose for humans is estimated at 800mg / kg. The substance has no mutagenic, teratogenic or carcinogenic effects. Carphedon administration to rats at 50mg / kg for two weeks resulted in increased ovarian weight and increased pregnancy index, but did not affect either pregnancy or embryo development [18].
Clinics
Carphedon has been or is being tested and recommended as a medicament in seven of indications such as:
a) CNS diseases of various etiology, especially in connection with vascular diseases and disorders of brain metabolism, intoxication, impaired mental function, decreased motor activity [2,3].
b) Prevention and therapy of stroke [19,20].
c) Neurotic disorders, neurocirculatory asthenia, flaccidity, increased fatigue, decreased psychomotor activity, attention deficit disorder, memory impairment [12,21,22].
d) Epilepsy [23-27].
e) Learning Disorders, Mild to Moderate Depression, Psychoorganic Syndrome, Mental Disorders, Apathetic Abuse Syndrome [1].
f) Prevention of hypoxia, increased stress resistance, correction of the functional state of the organism in extreme conditions by professionals to prevent the development of fatigue and improve mental and physical ability to work, correction of daily biorhythms [3,28,29].
g) Chronic alcoholism [30].
Contraindication
Contraindication is individual intolerance and allergy to pyrrolidine group drugs, pregnancy and breastfeeding. There is not enough clinical research data. It is also not recommended to prescribe carfedon to children because there is no data on their use in childhood. Caution should be exercised when administering carphedone to patients with severe organic liver and kidney damage, severe hypertension, and people with atherosclerosis. It should not be given to those who have a history of panic attacks or acute psychotic conditions, especially psychomotor agitation.
Side Effects
Insomnia, psychomotor restlessness, hyperemia of the skin, feeling warm, increasing blood pressure. The effects of carphedone can be potentiated by drugs that stimulate the CNS, antidepressants and nootropics.
Carphedon as Prohibited Doping
Even before carphedon was marketed as a nootropic with a similar effect to piracetam, it aroused the interest of athletes, especially athletes, as a new type of doping [31,32]. For the first time, carphedon discovered doping control of athletes at the Athletics World Championships in Athens in 1997 [33] and in 1998 the International Olympic Committee included it in the Prohibited List [34]. The World Anti-Doping Agency (WADA) leads it as a stimulant drug [35]. As an illicit doping, carphedon was first demonstrated in athletes at the 6th World Championships in Athletics, Athens 1997 [33,36]. Anti-doping analysis of about 100,000 urine samples from 2000 to 2009 by the World Anti-Doping Agency’s Italian Anti-Doping Laboratory showed 1.0 to 1.8% of positive findings. The most common were cannabinoids (0.2 - 0.4%), cocaine (0.1%) and stimulants - amphetamines, ephedrine, but also caffeine [37]. Carphedon is particularly popular among Russian athletes [38] and several have suffered from its use [39]. Among other things, the 2002 Olympic Biathlon Champion Olga Pylevová and the Russian cyclist Sergei Šilov [40].
Conclusion
Carphedon is classified by its pharmacological profile between nootropics and stimulants. Preclinical and partly clinical research has suggested that carphedon opens up new possibilities in the treatment of CNS diseases, but the results of clinical trials are not complete and not always entirely convincing. Carphedon abuse as doping and its inclusion in the Prohibited List limits its availability to official medicine and prevents it from being tested on humans again.
Acknowledgment
This work was supported by the long-term organization development plan (University of South Bohemia České Budějovice, Faculty of Health and Social Studies, České Budějovice, and University Hospital, Hradec Kralove, Czech Republic).
References
- Savchenko AY, Zakharova NS, Stepanov IN (2005) The phenotropil treatment of the consequences of brain organic lesions. Zh Nevrol Psikhiatr Im S S Korsakova 105(12): 22-26.
- Gustov AA, Smirnov AA, Korshunova Iu A, Andrianova EV (2006) Phenotropil in the treatment of vascular encephalopathy. [Article in Russian] Zh Nevrol Psikhiatr Im S S Korsakova 106(3): 52-53.
- Malykh AG, Sadaie MR (2010) Piracetam and piracetam-like drugs: From basic science to novel clinical applications to CNS disorders. Drugs 70(3): 287-312.
- Zvejniece L, Svalbe B, Veinberg G, Grinbergaq S, Vorona M, et al. (2011) Investigation into stereoselective pharmacological activity of phenotropil. Basic Clin Pharmacol Toxicol 109(5): 407-412.
- Firstova YY, Dolotov OV, Kondrakhin A, Dubynina EV, Grivennikov IA, et al. (2009) Effects of nootropic drugs on hippocampal and cortical BDNF levels in mice with different exploratory behavior efficacy. [Article in Russian] Eksp Klin Farmakol 72(6): 3-6.
- Khramova OV, Trofimov LA, Zkharov BE (2010) Efficacy and tolerance examination of phenotropil single-drug therapy in case of asthenic syndrome due to I and II stage chronic cerebral ischemia. Zdrov Celovek Severe 1: 12-16.
- Kim S, Park JH, Myung SW, Lho DS (1999) Determination of carphedon in human urine by solid-phase microextraction using capillary gas chromatography with nitrogen-phosphorus detection. Analyst 124(11): 1559-1562.
- Galesio M, Mazzarino M, de la Torre X, Botrè F, Capelo JL (2011) Accelerated sample treatment for screening of banned doping substances by GC-MS: ultrasonication versus microwave energy. Anal Bioanal Chem 399(2): 861-875.
- Kolmonen M, Leinonen A, Pelander A, Ojanperä I (2007) A general screening method for doping agents in human urine by solid phase extraction and liquid chromatography/time-of-flight mass spektrometry. Anal Chim Acta 585(1): 94-102.
- Bobkov YG, Morozov IS, Glozman OM, Nerobkova LN, Zhmurenko LA (1983) Pharmacological characteristics of a new phenyl analog of piracetam - 4-phenylpiracetam. [Article in Russian] Biull Eksp Biol Med 95(4): 50-53.
- Tiurenkov IN, Bagmetov MN, Epishina VV (2007) Comparative evaluation of the neuroprotective activity of phenotropil and piracetam in laboratory animals with experimental cerebral ischemia. Eksp Klin Farmakol 70(2): 24-29.
- Akarachkova ES (2011) Chronic fatigue and approaches to its treatment. Neurosci Behav Physiol 110(11 Pt 2): 48-54.
- Firstova YY, Abaimov DA, Kapitsa IG, Voronina TA, Kovalev GI (2011) The effects of scopolamine and the nootropic drug phenotropil on rat brain neurotransmitter receptors during testing of the conditioned passive avoidance task. Neurochem J 5(2):115-125.
- Samotrueva MA, Tyurenkov IN, Teplyi DL, Serezhnikova TK, Khlebtsova EB (2011) Psychoimmunomodulatoyry effect of phenotropil in Animals with Immune stress. Bull Exp Biol Med 151(1): 51-54.
- Maughan MT, Thevis Mario, Schänzer Wilhelm (2009) Performance-enhancing drugs. Olympic Textbook of Science in Sport. Wiley. USA.
- Zvejniece L, Svalbe B, Vavers E, Makrecka-Kuka M, Makarova E, et al. (2017) S-phenylpiracetam, a selective DAT inhibitor, reduces body weight gain without influencing locomotor activity. Pharmacol Biochem Behav 160: 21-29.
- Bugaeva LI, Spasov AA, Verovskij VE (2004) Functional-behavioral profile of new cyclic GABA analogs in acute toxicity tests. [Article in Russian] Eksp Klin Farmakol 67(3): 61-65.
- Khamidova TV, Chigirinskij I (2005) Effect of karphedone on reproduction function in female rats. [Article in Russian] Eksp Klin Farmakol 68(5): 29-31.
- Koval'chuk VV, Skoromets AA, Koval'chuk IV, Stoianova EG, Vysotskaia ML, et al. (2010) Efficacy of phenotropil in the rehabilitation of stroke patients. [Article in Russian] Zh Nevrol Psikhiatr Im S S Korsakova 110(12 Pt 2): 38-40.
- Voronina TA, Akhapkin RV (2011) Phenotropil for the prophylaxis and treatment of hemorrhagic stroke and acute phase of ischemic stroke. European Patent EP2011497, application number
- Kalinskij PP, Nazarov VV (2007) Use of phenotropil in the treatment of asthenic syndrome and autonomic disturbances in the acute period of mild cranial brain trauma. [Article in Russian] Zh Nevrol Psikhiatr Im S S Korsakova 107(2): 61-63.
- Fedin AI, Solov'eva ÉI, Mironova OP, Fedotova AV (2014) Treatment of asthenic syndrome in patients with chronic brain ischemia: results of the non-interventional observational program TRIUMPH. [Article in Russian] Zh Nevrol Psikhiatr Im S S Korsakova 114(12): 104-111.
- Bel'skaia GN, Ponomareva IV, Lukashevich IG, Tikhomirova IN (2007) Complex treatment of epilepsy with phenotropil. [Article in Russian] Zh Nevrol Psikh iatr Im S S Korsakova 107(8): 40-43.
- Lybzikova GN, Iaglova ZS, Kharlamova YS (2008) The efficacy of phenotropil in the complex treatment of epilepsy. [Article in Russian] Zh Nevrol Psikhiatr Im S S Korsakova 108: 69-70.
- Poverennova IE, Iakunina AV, Kalinin VA, Kurov MV (2011) Phenotropil in the complex treatment of symptomatic post traumatic epilepsy. Zh Nevrol Psikhiatr Im S S Korsakova 111(5 Pt 2): 81-83.
- Savenkov AA, Badalian OL, Avakian GN (2013) Nootropics and antioxidants in the complex therapy of symptomatic posttraumatic epilepsy. Zh Nevrol Psikhiatr Im S S Korsakova 113(6): 26-34.
- Grebeniuk OV, Zhukova NG, Alifirova VM (2014) The efficacy of add-on treatment with phenotropil in adult patients with locally-induced epilepsy. [Article in Russian] Zh Nevrol Psikhiatr Im S S Korsakova 114(11pt 2): 27-31.
- Novikova SG, Lobanova EG, Novikov DV, Emel'ianova TV (2008) Phenotropil use as premedication during out-patient stomatological treatment. Stomatologiia (Mosk) 87(3): 41-45.
- Liubimov AV (2013) The use of phenotropil in vertebrobasilar insufficiency. [Article in Russian] Zh Nevrol Psikhiatr Im S S Korsakova 113(12): 94-96.
- Staroverov AT, Zhukov OB, Raigorodskij YM (2011) Efficacy of transcranial magnetotherapy in the complex treatment of alcohol withdrawal syndrome. Neurosci Behav Physiol 39: 891-895.
- Grosse J, Thieme D, Lang R, Mueller RK (1999) Elucidation of Carphedon Doping - Designer Psychostimulant in Sports. In: W. Schänzer, H. Geyer, A. Gotzmann, U. Mareck-Engelke (eds.) Recent advances in doping analysis. Sport und Buch Strauß, Köln, pp. 349-359.
- Strano Rossi S, Abate MG, Braganò MC, Botrè F (2009) Use of stimulants and drugs of abuse in sport: the Italian experience. Adicciones 21(3): 239-242.
- Georgakopoulos CG, Tsitsimpikou C, Spyridaki MHE, Lyris E, Cookeas EG, et al. (1999) Doping control analysis: the 6th World Championship of Athletics, Athens, Greece. TrAC Trends Anal Chem 18: 1-13.
- IOC (2003) The IOC list of prohibited classes of substances and methods 2003. Dostupné Canada.
- Docherty JR (2008) Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA). Brit J Pharmacol 154: 606-622.
- Laure P, Lecerf T, Friser A, Binsinger C (2004) Drugs, recreational drug use and attitudes towards doping of high school athletes. Int J Sports Med 25(2): 133-138.
- Strano Rossi S, Botrè F (2011) Prevalence of illicit drug use among the Italian athlete population with special attention on drugs of abuse: a 10-year review. J Sports Sci 29(5): 471-476.
- Tsoutsoulova-Draganova A, Halatcheva N, Kurteva V, Carova D, Andreeva A, et al. (1999) Investigations on a “Black Market’s Drug - Carphedon“. In: W Schänzer, H Geyer, A Gotzmann, U Mareck-Engelke (eds.) Recent advances in doping analysis. Sport und Buch Strauß, Köln 475-482.
- Semenov VA, Bolotov SL, Sizoi VF (1999) New Stimulant of Russia-Carphedon. In: W Schänzer, H Geyer, A Gotzmann, U Mareck-Engelke (eds.) Recent advances in doping analysis. Sport und Buch Strauß, Köln 337-348.
- (2009) Ruský cyklista Šilov dostal dvouletý trest za doping. Dostupné z: Sport.cz.






























