Phytochemical Characterization and Antioxidative Property of Ocimum canum Sims
R Kuralarasi1,S Revathilakshmi2*
1Research scholar, Ayya Nadar Janaki Akmal College, India
2Department of Botany, Ayya Nadar Janaki Akmal College, India
*Corresponding author: S Revathilakshmi, Assistant professor, Department of Botany, Ayya Nadar Janaki Akmal College, Sivakasi-626124, India
How to cite this article:R Kuralarasi, S Revathilakshmi. Phytochemical Characterization and Antioxidative Property of Ocimum canum Sims. Glob J Pharmaceu Sci. 2019; 7(3): 555712. DOI: 10.19080/GJPPS.2019.06.555712.
Abstract
Medicinal plants are the richest bio-resource of drugs for traditional systems of medicine, modern medicine, nutraceuticals, food supplements, folk medicines, pharmaceutical intermediates and chemical entities for synthetic drugs. In the present study aimed to find out the phytochemicals present in the various solvent leaf extracts of Ocimum canum Sims and all the fractions of O. canum demonstrated H-donor activity. The highest DPPH radical scavenging activity was detected in chloroform fraction (IC50 0.145mg/ml), followed by ethyl acetate, pet-ether and residual fractions (IC50 0.164, 0.29 and 0.6mg/ml respectively). The residual fraction of O. canum showed the highest reducing ability (absorbance 0.620) than all the other fractions tested. The scavenging activity of the pet-ether fraction (IC50 0.141mg/ml) was higher than that of quercetin (0.308mg/ml). The residual, chloroform and ethyl acetate fractions also showed significant scavenging activities (IC50 of RF, CF and EAF were 0.125, 0.186 and 0.232mg/ml respectively) when compared to the standard.
The chloroform fraction showed strong nitric oxide scavenging activity (IC50 0.183mg/ml) and that of standard curcumin was 0.078mg/ml. The total antioxidant activity of the fractions of O. canum was determined by the thiocyanate method and compared with the standard, a-tocopherol. The phosphomolybdate method is quantitative, since the total antioxidant capacity is expressed as α-tocopherol equivalents. Among the fractions tested, the chloroform fraction showed the highest ferrous ion chelating ability (IC50 0.276mg/ml). Moreover, total phenolics concentration equivalents to gallic acid was found in the range of 59.50 to 109.0mg/g of plant extracts, which correlated with antioxidant activity. The findings indicated promising antioxidant activity of crude extracts of the above plants and needs further exploration for their effective use in both modern and traditional system of medicines.
Keywords: Phytochemical; Ocimum canum; Antioxidative
Introduction
Medicinal plants are commonly rich in phenolic compounds, such as flavonoids, phenolic acids, stilbenes, tannins, coumarins, lignans and lignins. These compounds have multiple biological effects including antioxidant activity. Antioxidants are widely used in dietary supplements and have been investigated for the prevention of diseases such as cancer, coronary heart disease and even altitude sickness. Although initial studies suggested that antioxidant supplements might promote health Dabelstein et al. [1]. Antioxidants or inhibitors of oxidation are compounds which retard or prevent the oxidation and in general prolong the life of the oxidizable matter Kokate et al. [2]. Free radicals are constantly generated resulting in extensive damage to tissues and biomolecules leading to various disease conditions. So, the medicinal plants with antioxidant property are employed as an alternative source of medicine to mitigate the diseases associated with oxidative stress Nithya and Balkrishnan etal. [3]. Free radicals cause many human diseases like cancer, Alzheimer’s disease, cardiac reperfusion abnormalities, kidney disease and fibrosis etc. Antioxidants play many vital functions in a cell and have many beneficial effects when present in foods Sharma et al. [4]. The Ocimum canum belongs to the Lamiaceae. It is commonly known as Nai tulasi is a rich source of aromatic compounds. These compounds are being used as antimicrobial, antiemetic, antidiabetic, antifertility, antiasthmatic, antistress and anticancer activity [5]. The plant shows a pungent, aromatic flavour and is commonly cultivated for culinary purposes. O. canum is used specially for treating various types of diseases and lowering blood glucose and treats cold, fever, parasiticinfestations on the body and inflammation of joints and headaches Ngassoum et al. [6]. The findings indicated promising antioxidant activity of crude extracts of the above plants and needs further exploration for their effective use in both modern and traditional system of medicines.
Material and Methods
Collection and Extraction of Plant
Fresh O. canum plants were collected from the Botanical garden of Ayya Nadar Janaki Ammal College, Sivakasi. Leaves were separated from the plant and dried under shade. Then the leaves were powdered.
Preparation of the Extract and Fractionation
The air-dried powdered leaves of O. canum (100g) was extracted with methanol-water (7:3) mixture using a mechanical shaker for 4h. The resultant extract was concentrated under reduced pressure to yield a residue. The hydromethanolic extract was then extracted successively with equal volumes of petroleum-ether, chloroform and ethyl acetate. Each fraction was then concentrated under reduced pressure to obtain the pet-ether fraction (PEF1.6%w/w), chloroform fraction (CF- 1.8%w/w), ethyl acetate fraction (EAF-0.8%w/w) and residual fraction (RF5.3%w/w).
Drugs and Chemicals
DPPH was obtained from Hi Media Laboratories Pvt. Ltd., Mumbai. Quercetin and pyrocatechol were purchased from Sisco Research Laboratories Pvt. Ltd., Mumbai. Folin-Ciocalteu reagent was obtained from SD Fine Chemicals Pvt. Ltd, Mumbai. Calf thymus DNA was purchased from Genei Chemicals, Bangalore. Ferrozine was obtained from Sigma Aldrich, USA. All other drugs and chemicals used in the study were obtained commercially and were of analytical grade.
Phytochemical Characterization
Leaf samples of O. canum extracted with different solvents were subjected to various preliminary phytochemical analysis for the presence or absence of various phytoconstituents by the following tests adapted by Rai et al. [7].
In vitro Antioxidant Activity
The free radical scavenging activity of the fractions was measured in vitro by 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay [8]. The reducing power was investigated by the Fe3+-Fe2+ transformation in the presence of the fractions as described by Fejes et al. [9]. The Fe2+ can be monitored by measuring the formation of Perl’s Prussian blue at 700nm [10]. Hydroxyl radical scavenging activity was measured by the ability of the different fractions of O. canum to scavenge the hydroxyl radicals generated by the Fe3+-ascorbate-EDTA-H2O2 system, Fenton reaction [11,12]. The percentage scavenging activity at different concentrations of the fractions was determined and the IC50 values were compared with the standard, a-tocopherol [13]. Nitric oxide radical scavenging assay was performed according to the method described by Sreejayan et al. [14].
The peroxy radical scavenging activity was determined by thiocyanate method using a- tocopherol (50-800μg/ml) as standard [15]. The total antioxidant capacity of the fractions was determined by phosphomolybdate method using α-tocopherol as the standard [16]. The percentage chelating effect on ferrozine- Fe2+ complex was calculated. The IC50 values were compared with ascorbic acid [17]. The pro-oxidant activity of the fractions was determined by bleomycin-dependent DNA damage. All the determinations were carried out in triplicate [18]. Total soluble phenolics of the fraction were determined with Folin- Ciocalteu reagent using pyrocatechol as the standard [19]. Total soluble flavonoid content of the fractions was determined with aluminium nitrate using quercetin as the standard [20].
Statistical Analysis
All experiments were performed in triplicate (n=3) and results were expressed as mean ± SE. Statistical analysis was carried out with (SPSS package version 17.10) using ANOVA followed by Turkey’s test (P<0.05).
Results
Phytochemical Screening
Phytochemical screening of the crude extracts of different fractions of the leaves of Ocimum canum was represented in (Table 1). Carbohydrates were observed in the alcoholic and aqueous extracts of leaves whereas phytosterols were present in the chloroform and petroleum ether extracts of leaves. Volatile oils showed their presence when the leaves are extracted with chloroform and petroleum ether. Tannins were observed in the alcoholic and chloroform extracts. Flavonoids showed their presence when the leaves were extracted with either alcohol or water. Terpenoids were present in the chloroforms and petroleum ether leaf extracts. Alkaloids, saponins, glycosides, gums and mucilage were not observed in any of the solvent extracts examined.
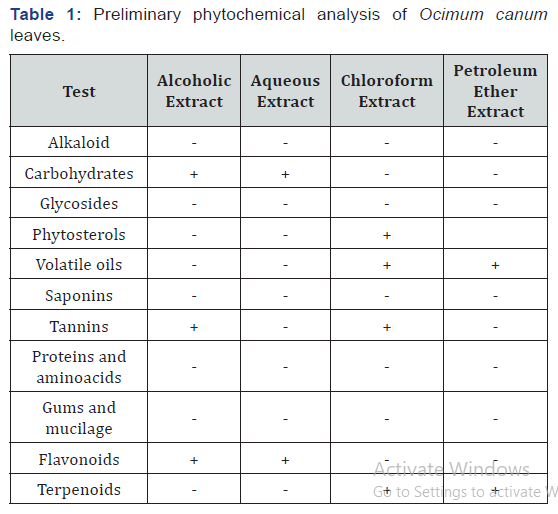
DPPH Assay
All the fractions of O. canum demonstrated H-donor activity. The highest DPPH radical scavenging activity was detected in chloroform fraction (IC50 0.145mg/ml), followed by ethyl acetate, pet-ether and residual fractions (IC50 0.164, 0.29 and 0.6mg/ml respectively) (Table 2). These activities are less than that of ascorbic acid. The scavenging ability increased towards the ethyl acetate fraction with increasing polarity of the solvent.
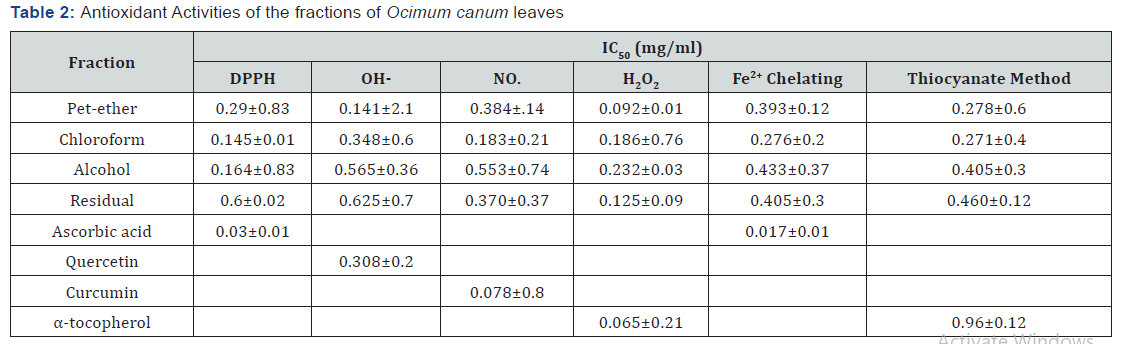
Reducing Power Ability
(Table 3) shows the reductive capabilities of different fractions of O. canum when compared to the standard, BHT. Like the antioxidant activity, the reducing power increased with increasing amount of the fractions. The residual fraction of O. canum showed the highest reducing ability (0.620nm) than all the other fractions tested. However, the activity was less than the standard, BHT (1.092nm). The pet-ether, chloroform and ethyl acetate fractions also showed significant activity indicating its reductive ability.
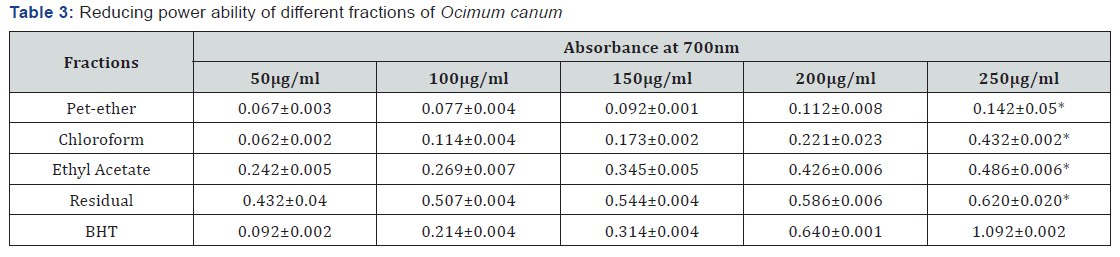
Hydroxyl Radical Scavenging Assay
Hydroxyl radical scavenging activity was quantified by measuring the inhibition of the degradation of deoxyribose by the free radicals generated by the Fenton reaction. All the fractions of O. canum and the standard (quercetin) inhibited the production of hydroxyl radicals. The scavenging activity of the pet-ether fraction (IC50 0.141mg/ml) was higher than that of quercetin (0.308 mg/ml). The IC50s values of the chloroform, ethyl acetate and residual fractions were 0.348, 0.565 and 0.625mg/ml respectively (Table 2).
Hydrogen Peroxide Scavenging Assay
All the fractions of C. grandis scavenged hydrogen peroxide in a concentration-dependent manner. The pet-ether fraction (PEF) of O. canum showed strong H2O2 scavenging activity (IC50 0.092mg/ml) whereas that of the standard, a-tocopherol was 0.065mg/ml. The residual, chloroform and ethyl acetate fractions also showed significant scavenging activities (IC50 of RF, CF and EAF were 0.125, 0.186 and 0.232mg/ml respectively) when compared to the standard (Table 2).
Nitric Oxide Radical Scavenging Assay
The fractions of O. canum effectively reduced the generation of nitric oxide from Sodium nitroprusside. The chloroform fraction showed strong nitric oxide scavenging activity (IC50 0.183mg/ml) and that of standard curcumin was 0.078mg/ml. The residual fraction (0.37mg/ml), pet-ether fraction (0.384mg/ ml) and ethyl acetate fraction (0.553mg/ml) also showed good scavenging activities (Table 2).
Thiocyanate Method
The total antioxidant activity of the fractions of O. canum was determined by the thiocyanate method and compared with the standard, a-tocopherol. The absorbance decreased with the increasing concentrations of the fractions, which indicate that the fractions could effectively decrease the amount of formed peroxides. The total antioxidant activity of the pet-ether and chloroform fractions were almost similar (IC50,/ 0.278 and 0.271mg/ml respectively) and that of the standard, α-tocopherol was 0.096mg/ml. The ethyl acetate and residual fractions also showed good antioxidant activity but at higher concentrations (IC50 0.405 and 0.46mg/ml respectively) (Table 2).
Phosphomolybdate Method
The phosphomolybdate method is quantitative, since the total antioxidant capacity is expressed as α-tocopherol equivalents. Among the fractions tested, the chloroform fraction contains 31.66μg vitamin E equivalent/ 100μg. The antioxidant activity increased in the order of chloroform fraction > residual fraction > ethyl acetate fraction > pet-ether fraction (Table 4).
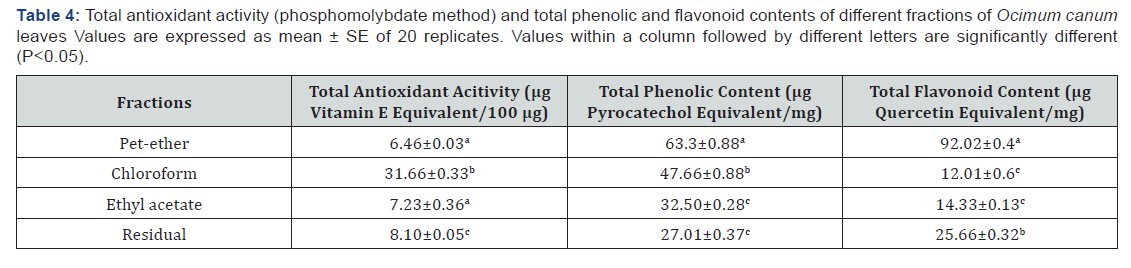
Ferrous Chelating ability
Addition of the fractions of O. canum interferes with the ferrous-ferrozine complex and the red colour of the complex decreased with the increasing concentrations of the fractions. All the fractions captured ferrous ions before ferrozine and thus have ferrous chelating ability. Among the fractions tested, the chloroform fraction showed the highest ferrous ion chelating ability (IC50 0.276mg/ml). The abilities shown by pet-ether, ethyl acetate and residual fractions were almost similar (IC50 0.393, 0.433 and 0.405mg/ml respectively). Ascorbic acid (IC50 0.017mg/ml) showed the highest ferrous ion chelating ability (Table 2).
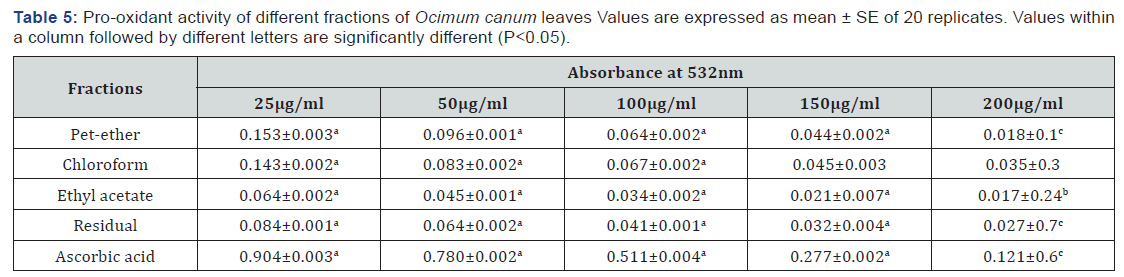
Bleomycin-Dependent DNA Damage
The pro-oxidant activity of the fractions of C. grandis was assessed by their effects on damage to DNA in the presence of a bleomycin-Fe3+ complex. The absorbance of all the fractions decreased with increasing concentrations, which proves that none of the fractions exhibited pro-oxidant activity (Table 5).
Total Phenolic and Flavonoid Content
Total phenolic content was estimated by using Folin- Ciocalteu reagent. Total phenolic content of the different fractions of O. canum were solvent dependent and expressed as μg pyrocatechol equivalent. The content of the total phenolics in the fractions decreased in the order of pet-ether > chloroform > ethyl acetate > residual fractions. The total flavonoid content in the fractions was expressed as μg quercetin equivalent. The petether fraction of O. canum showed highest number of flavonoids among the fractions tested. The content of total flavonoids in the fractions decreased in the order of pet-ether fraction > residual fraction > ethyl acetate fraction > chloroform fraction (Table 4).
Discussion
Free radicals are known to play a definite role in a wide variety of pathological manifestations. Antioxidants fight free radicals and protect us from various diseases. They exert their action either by scavenging the reactive oxygen species or protecting the antioxidant defence mechanisms. DPPH assay is one of the most widely used methods for screening antioxidant activity of plant extracts [21]. DPPH is a stable, nitrogen-centered free radical which produces violet colour in ethanol solution. It was reduced to a yellow coloured product, diphenylpicryl hydrazine, with the addition of the fractions in a concentration-dependent manner. The reduction in the number of DPPH molecules can be correlated with the number of available hydroxyl groups. All the fractions showed significantly higher inhibition percentage and positively correlated with total phenolic content.
The transformation of Fe3+ into Fe2+ in the presence of various fractions was measured to determine the reducing power ability. The reducing ability of a compound generally depends on the presence of reductions (antioxidants), which exert the antioxidant activity by breaking the free radical chain by donating a hydrogen atom [10]. The antioxidant principles present in the fractions of O. canum caused the reduction of Fe3+/ ferricyanide complex to the ferrous form, and thus proved the reducing power ability.
Hydroxyl radical is the most deleterious and reactive among the ROS and it bears the shortest half-life compared with other free radicals. The oxygen derived hydroxyl radicals along with the added transition metal ion (Fe2+) causes the degradation of deoxyribose into malondialdehyde which produces a pink chromogen with thiobarbituric acid [11]. All the fractions of O. canum when added to the reaction mixture, scavenged the hydroxyl radicals and prevented the degradation of deoxyribose.
Hydrogen peroxide itself is not particularly reactive with most biologically important molecules but is an intracellular precursor of hydroxyl radicals which is very toxic to the cell Halliwell. Thus, scavenging of H2O2 is a measure of the antioxidant activity of the fractions. All the fractions of O. canum scavenged hydrogen peroxide which may be attributed to the presence of phenolic groups that could donate electrons to hydrogen peroxide, thereby neutralising it into water.
In vitro inhibition of nitric oxide radical is a measure of antioxidant activity of plant drugs. Nitric oxide is a free radical which plays an important role in the pathogenesis of pain, inflammation, etc. Scavenging of nitric oxide radical is based on the generation of nitric oxide from Sodium nitroprusside in buffered saline, which reacts with oxygen to produce nitrite ions that can be measured by using Griess reagent [22]. The absorbance of the chromophore is measured at 546nm in the presence of the fractions. All the fractions of O. canum decreased the amount of nitrite generated from the decomposition of sodium nitroprusside in vitro. This may be due to the antioxidant principles in the fractions which compete with oxygen to react with NO· thereby inhibiting the generation of nitrite.
The amount of formed peroxides was measured by the thiocyanate method. The fractions were incubated with linoleic emulsion in dark at 37°C and the amount of peroxides was determined spectrophotometrically by measuring the absorbance at 500nm [23]. A decrease in absorbance indicated the antioxidant activity of the fractions which might be due to the inactivation of the free radicals and the presence of flavonoid like phytochemicals.
The phosphomolybdate method has been routinely used to evaluate the total antioxidant capacity of the extracts [23]. In the presence of the fractions, the Mo (VI) is reduced to Mo(V) and forms a green coloured phosphomolybdenum V complex which shows maximum absorbance at 695nm. All the fractions possessed antioxidant activity.
The metal chelating ability of the fractions of O.canum was measured by the formation of ferrous ionferrozine complex. Ferrozine combines with ferrous ions forming a red coloured complex which absorbs at 562nm [24]. It was reported that the chelating agents which forms bond with a metal, are effective as secondary antioxidants, because they reduce the redox potential thereby stabilising the oxidised form of the metal ion [25]. The results of our study demonstrate that the fractions have an effective capacity for iron binding, suggesting its antioxidant potential. In addition, the metal chelating ability of the fractions demonstrated that they reduce the concentration of the catalysing transition metal involved in the peroxidation of lipids.
Bleomycin-dependent DNA damage has been adopted as a sensitive and specific method to examine the potential prooxidant drugs. Degradation of DNA occur if the samples to be tested reduce the bleomycin-Fe3+ to bleomycin-Fe2+ resulting in the formation of a product like MDA which reacts with TBA to give a pink colour [26]. All the fractions decreased the absorbance and bleomycin-Fe3+ is not converted into bleomycin Fe2+, thereby preventing the DNA degradation. These results confirm that the fractions of O. canum are devoid of pro-oxidant activity.
Phenolics are ubiquitous secondary metabolites in plants and possess a wide range of therapeutic uses such as antioxidant, antimutagenic, anticarcinogenic, free radical scavenging activities and decrease cardiovascular complications [27,28]. The scavenging ability of the phenolics is mainly due to the presence of hydroxyl groups. Total phenolic assay by using Folin-Ciocalteu reagent is a simple, convenient and reproducible method. It is employed routinely in studying phenolic antioxidants [29]. Flavonoids are a group of polyphenolic compounds, which exhibit several biological effects such as antiinflammatory, antihepatotoxic, antiulcer, antiallergic, antiviral, anticancer activities. They also inhibit enzymes such as aldose reducatse and xanthine oxidase [30,31].
They are capable of effectively scavenging the reactive oxygen species because of their phenolic hydroxyl groups and are potent antioxidants [23]. In view of their wide pharmacological and biological actions, they have a greater therapeutic potential. The presence of high phenolic and flavonoid content in the fractions has contributed directly to the antioxidant activity by neutralising the free radicals. Based on the results obtained, it may be concluded that all the fractions of the leaves of O. canum showed strong antioxidant activity, reducing power ability, free radical scavenging activity, metal chelating ability and inhibition of ß-carotene bleaching when compared to standards such as ascorbic acid, α-tocopherol, curcumin, and butylated hydroxytoluene [32]. As the various fractions of O. canum exhibited different reactive oxygen species scavenging activities, there may be different percentages of phytochemical constituents present in the fractions. Further studies to evaluate the in vivo potential of the fractions in various animal models and the isolation and identification of the antioxidant principles in the leaves of Ocimum canum are being carried out.
References
- Dabelstein, Reglitzky, Schütze, Reders (2007) Automotive Fuels. Ullmann's Encyclopedia of Industrial Chemistry 1(2): 16-719.2.
- Kokate CK, Purohit AP (2004) Textbook of pharmacognosy. 29: 542.
- Narayanaswamy N, Balkrishnan KP (2011) Evaluation of some medicinal plants for their antioxidant properties. IJPRIF 1(3): 381-385.
- Sharma SK, Singh L, Singh S (2013) A review on medicinal plants having antioxidant potential. Indian J Res Pharm Biotechnic 1(3): 404-409.
- Nangia-Makker P1, Tait L, Shekhar MP, Palomino E, Hogan V, et al. (2007) Inhibition of breast tumor growth and angiogenesis by a medicinal herb Ocimum gratissimum. Int J Cancer 121(4): 884–894.
- Ngassoum MB, Ousmaila H, Ngamo LT, Maponmetsem PM, Jirovetz L, et al. (2004) Aroma compounds of essential oils of two varieties of the spice plant Ocimum canum From northern Cameroon. J Food Comp Anal 17(2): 197-204.
- Rai V, Ramanath VP, Pratapchandra K (2016) A preliminary evaluation of anticancer and antioxidant potential of two traditional medicinal plants from Lamiaceae-Pogostemon heyneanus and Plectranthus amboinicus. J App Pharma Sci 6(8): 73-78.
- Mensor LL, Menezes FS, Leitão GG, Reis AS, dos Santos TC, et al. (2001) Screening of Brazilian plants extracts for antioxidants activity by the use of DPPH free radical method. Phytother Res 15(2): 127-130.
- Fejes S, Blázovics A, Lugasi A, Lemberkovics E, Petri G, et al. (2000) In vitro antioxidant activity of Anthriscus cerefolium L (Hoffm.) extracts. J Ethnopharmacol 69(3): 259-265.
- Meir S, Kanner J, Akiri B, Hadar SP (1995) Determination and involvement of aqueous reducing compounds in oxidative systems of various senescing leaves. J Agric Food Chem 43(7): 1813-1817.
- Halliwell B, Gutteridge JM, Aruoma OI (1987) The deoxyribose method: a simple test tube assay for the determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165(1): 215-219.
- Ilavarasa006E R, Mallika M, Venkataraman S (2005) Anti-inflammatory and antioxidant activities of Cassia fistula bark extracts. Afr J Trad CAM 2(1): 70-85.
- Oktay M, Gulcin I, Kufrevioglu O (2003) Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm Wiss U Technol 36(2): 263-271.
- Sreejayan, Rao MN (1997) Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol 49(1): 105-107.
- Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269(2): 337-341.
- Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK (2002) Evaluation of antioxidant activities and antimutagenicity of turmeric oil: A byproduct from curcumin production. Z Naturforsch 57(9-10): 828-835.
- Huang SC, Kuo JC (2000) Concentrations and antioxidant activity of Anserine and Carnosine in poultry meat extracts treated with demineralization and papain. Proc Natl Sci Counc ROC (B) 24(4): 193-201.
- Ng TB, Liu F, Lu Y, Cheng CH, Wang Z (2003) Antioxidant activity of compounds from the medicinal herb Aster tataricus. Compar Biochem Physiol Part C 136(2): 109-115.
- Gülçin I, Oktay M, Küfrevioğlu OI, Aslan A (2002) Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol 79(3): 325-329.
- Hsu CY (2006) Antioxidant activity of extract from Polygonum aviculare Biol Res 39(2): 281-288.
- Nanjo F, Goo K, Set R, Suzuki M, Sakai M, et al. (1996) Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picryl hydrazyl radical. Free Radic Biol Med 21(6): 895-902.
- Marcocci L, Packer L, Droy Lefaix MT, Sekaki A, Gardès Albert M (1994) Antioxidant action of Ginkgo biloba extracts EGP761. Methods Enzymol 234: 462-475.
- Duh PD, Tu YY, Yen (1999) Antioxidant activty of water extract of harng Jyur (Chrysanthemum morifolium Ramat). Lebens Wiss U Technol 32: 269-277.
- Cao G, Sofic E, Prior RL (1997) Antioxidant and pro-oxidant behaviour of flavonoids: structure activity relationships. Free Radic Biol Med 22(5): 749-760.
- Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H (2000) Antioxidant and antiglycation of carcinol from Garcina indica fruit rind. J Agric Food Chem 48(2): 180-185.
- Liu F, Ng TB (2000) Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci 66(8): 725-735.
- Gow Chin Yen, Pin Der Duh, Charing Liang Tsai (1993) Relationship between antioxidant activity and maturity of peanut hulls. J Agric Food Chem 41(1): 67-70.
- Gow-Chin Yen, Hui-Yin Chen (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem 43(1): 27-32.
- Dejian Huang, Boxin Ou, Ronald L Prior (2005) The chemistry behind antioxidant capacity assays. J Agricul Food Chem 53(6): 1841-1856.
- Bassolé IH, Guelbeogo WM, Nébié R, Costantini C, Sagnon N, et al. (2003) Ovicidal and Larvicidal activity against Aedes aegypti and Anopheles gambiae complex mosquitoes of essential oils extracted from three spontaneous plants of Burkina Faso. Parasitol 45(1): 23–26.
- Panchawat S, Rathore KS, Sisodia SS (2010) A review on herbal antioxidants. Int J Pharma Tech Res (1): 232-239.
- Yildrim, Oktay, Bilaloglu, V (2001) The antioxidant activity of the leaves of Cydonia vulgaris. Turk J Med Sci 31: 23-27.






























