Complementary and Alternative Medicine: Evaluation of the Physicochemical and Thermal Properties of the Biofield Energy Treated Metronidazole
Alice Branton1, Mahendra Kumar Trivedi1, Dahryn Trivedi1, Gopal Nayak1 and Snehasis Jana2*
1Trivedi Global, Inc., Henderson, USA
2Trivedi Science Research Laboratory Pvt. Ltd., Bhopal, India
Submission: March 13, 2019; Published: April 09, 2019
*Corresponding author: Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd., Bhopal, India
How to cite this article: Alice B, Mahendra K T, Dahryn T, Gopal N, Snehasis J. Complementary and Alternative Medicine: Evaluation of the Physicochemical and Thermal Properties of the Biofield Energy Treated Metronidazole. Glob J Pharmaceu Sci. 2019; 7(2): 555709. DOI: 10.19080/GJPPS.2019.06.555709.
Abstract
Metronidazole is an antibiotic and is useful for antibacterial and antiprotozoal medication. In this research work, the impact of the Trivedi Effect® (Consciousness Energy Healing Treatment) on the physicochemical properties of metronidazole was evaluated using the modern analytical technique. Metronidazole sample was divided into two parts, one part of metronidazole was considered as a control sample (no Biofield Energy Treatment was provided); however, the other part of metronidazole, was exposed to the Consciousness Energy Healing Treatment remotely by a well-known Biofield Energy Healer, Alice Branton and termed as a treated sample. The peak intensities, as well as crystallite sizes of the treated metronidazole were significantly altered ranging from -97.25% to 463% and -83.18% to 123.79%, respectively; however, the average crystallite size was significantly decreased by 14.91% compared with the control sample. The particle size values in the treated metronidazole were significantly decreased by 14.69%(d10), 10.82%(d50), 19.14%(d90), and 16.88% {D (4,3)}; thus, the specific surface area was significantly increased by 24.7% compared to the control sample. The latent heat of fusion and latent heat of decomposition were decreased by 2.1% and 9.41%, respectively in the treated sample compared with the control sample. The total weight loss was increased by 2.56%; however, the residue amount was significantly decreased by 83.86% in the treated sample compared with the control sample.
The maximum thermal degradation temperature was decreased by 3.53% in the treated sample compared with the control sample. Thus, the Consciousness Energy Healing Treatment might have generated a new polymorphic form of metronidazole which may offer better solubility, dissolution, and good bioavailability compared with the control sample. The Consciousness Energy Treated metronidazole would be very useful to design more efficacious pharmaceutical formulations for the better therapeutic response against bacterial and protozoal infection in the vagina, stomach, liver, skin, joints, brain, and respiratory tract, aspiration pneumonia, rosacea, fungating wounds, intra-abdominal infections, lung abscess, periodontitis, amoebiasis, oral infections, etc.
Keywords: Complementary and Alternative Medicine; Metronidazole; The Trivedi Effect®; Consciousness Energy Healing Treatment; PXRD; Particle size; Surface area; DSC; TGA/DTG
Introduction
Metronidazole is the nitroimidazole class of antibiotic and useful for the antiprotozoal medication. It inhibits the microorganism by disrupting the DNA of microbial cells for the nucleic acid synthesis. It has the relatively little effect on human cells or aerobic bacteria, but this function only occurs when metronidazole is partially reduced, which usually happens only in anaerobic cells [1,2]. It is used to treat the bacterial infections of the vagina (bacterial vaginosis), stomach (giardiasis, pseudomembranous colitis), liver, skin, joints (pelvic inflammatory disease), brain, and respiratory tract, aspiration pneumonia, rosacea, fungating wounds, intra-abdominal infections, lung abscess, periodontitis, amoebiasis, oral infections, and infections caused by susceptible anaerobic organisms such as Bacteroides, Clostridium, Fusobacterium, Dracunculus, Peptostreptococcus, Helicobacter pylori, and Prevotella species, etc. [2-5]. It is also used for the infections of Giardia in cats, dogs, horse, and other companion animals [2,6].
Common side effects associated with the metronidazole therapy are nausea, vomiting, headache, dizziness, diarrhoea, weight loss, abdominal pain, metallic taste in the mouth, thrombophlebitis, hypersensitivity reactions, stomatitis, glossitis, dark urine,leucopenia, neutropenia, peripheral neuropathy, central nervous system toxicity, and paraesthesia etc. [2,7]. Metronidazole is bitter, and so in the liquid suspension, it contains in the form of metronidazole benzoate. Metronidazole has high oral bioavailability. It is also delivered in the form of the tablet, capsule, and intravenous injection also [7-9]. It is very harmful in case of skin contact (irritant, permeator), eye contact (irritant), inhalation, and ingestion. The solubility profile of metronidazole is very poor, where is slightly soluble in cold water, hot water, alcohol, chloroform, dilute acid, and dimethylformamide [10,11].
Many scientific communities throughout the globe doing the research work for the improvement of better physicochemical properties of the nutraceutical and pharmaceutical compounds, because the physicochemical properties of the pharmaceutical or nutraceutical compounds play a crucial role in its dissolution, absorption, and bioavailability profile in the body [12]. In this scenario, the Trivedi Effect®-Biofield Energy Healing Treatment has the significant impact on the physicochemical properties such as particle size, surface area, thermal behaviour, and bioavailability profile of nutraceutical and pharmaceutical compounds [13-17]. The Trivedi Effect® is a natural and only scientifically confirmed phenomenon in which a person can harness this inherently intelligent energy from the “Universe” and transmit it anywhere on the planet through the possible mediation of neutrinos [18].
This unique energy field exists surrounding the body of every living organism called the “Biofield”, which is infinite and para- dimensional electromagnetic field. The Biofield Energy Healing Therapies have been testified with significantly beneficial outcomes against various disease conditions [19]. The National Institutes of Health (NIH) and National Center for Complementary and Alternative Medicine (NCCAM) recommend and included the Energy Therapy under the Complementary and Alternative Medicine (CAM) along with homeopathy, traditional Chinese herbs and medicines, aromatherapy, Qi Gong, Tai Chi, Reiki, hypnotherapy, Ayurvedic medicine, yoga, chiropractic/osteopathic manipulation, massage, relaxation techniques, meditation, etc., which has been accepted by most of the U.S. people [20,21].
The Trivedi Effect®-Consciousness Energy Healing Treatment has the significant potential for the transformation of the object( s), and the outcomes were published in numerous scientific journals. The Biofield Energy Treatment (the Trivedi Effect®) has the amazing capability to transform the physicochemical, structural, and behavioural properties of metals and ceramics [22,23], organic compounds [24,25], nutraceuticals [26,27], pharmaceuticals [28,29], microorganisms [30,31], various living cells [32,33], and improve the overall productivity of crops [34,35]. Therefore, the current study was designed and evaluated the impact of the Consciousness Energy Healing Treatment on the physicochemical and thermal properties of metronidazole using powder X-ray diffraction, particle size analysis, differential scanning calorimetry, and thermogravimetric analysis/ differential thermogravimetric analysis.
Materials and Methods
Chemicals and Reagents
Metronidazole (2-Methyl-5-nitroimidazole-1-ethanol) was purchased from Tokyo Chemical Industry Co., Ltd., Japan and other chemicals were purchased from India.
Consciousness Energy Healing Treatment Strategies
Metronidazole was the test sample for the experiment, which further divided into two equal parts. One part of metronidazole was treated with the Energy of Consciousness Healing Treatment (the Trivedi Effect®) remotely under standard laboratory conditions for 3 minutes by the well-known Biofield Energy Healer, Alice Branton, USA, and known as the Biofield Energy Treated metronidazole sample. However, the second part of metronidazole was considered as a control sample (no Biofield Energy Treatment was provided). Further, the control sample was treated with a “sham” healer, who did not have any knowledge about the Biofield Energy Treatment. After treatment, both the samples were kept in the sealed conditions and characterized using modern analytical techniques.
Characterization
The PXRD, PSA, DSC, and TGA analysis of metronidazole were performed. The PXRD analysis of metronidazole powder sample was performed with the help of Rigaku MiniFlex-II Desktop X-ray diffractometer (Japan) [36,37]. The average size of crystallites was calculated from PXRD data using the Scherrer’s formula (1)
Where G is the crystallite size in nm, k is the equipment constant (0.94), λ is the radiation wavelength (0.154056 nm for Kα1 emission), β is the full-width at half maximum, and θ is the Bragg angle [38].
The PSA was performed using Malvern Mastersizer 2000, from the UK with a detection range between 0.01 μm to 3000 μm using the wet method [39,40]. Similarly, the DSC analysis of metronidazole was performed with the help of DSC Q200, TA instruments. The TGA/DTG thermograms of metronidazole were obtained with the help of TGA Q50 TA instruments [39,40].
The % change in crystallite size, peak intensity, particle size, specific surface area (SSA), peak intensity, melting point, latent heat, weight loss and the maximum thermal degradation temperature (Tmax) of the Biofield Energy Treated sample was calculated compared with the control sample using the following equation 2:
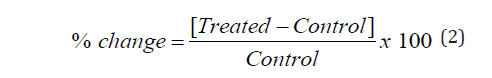
Results and Discussion
The powder XRD diffractograms of both the sample metronidazole showed sharp and intense peaks (Figure 1) indicated that both the samples were crystalline. Both the samples showed the highest peak intensity at 2θ equal to 12.6° (Table 1, entry 2). The peak intensities of the Biofield Energy Treated metronidazole were significantly altered compared to the control sample. Overall, the peak intensities of the Biofield Energy Treated metronidazole were significantly altered ranging from -97.25% to 463% compared to the control sample.
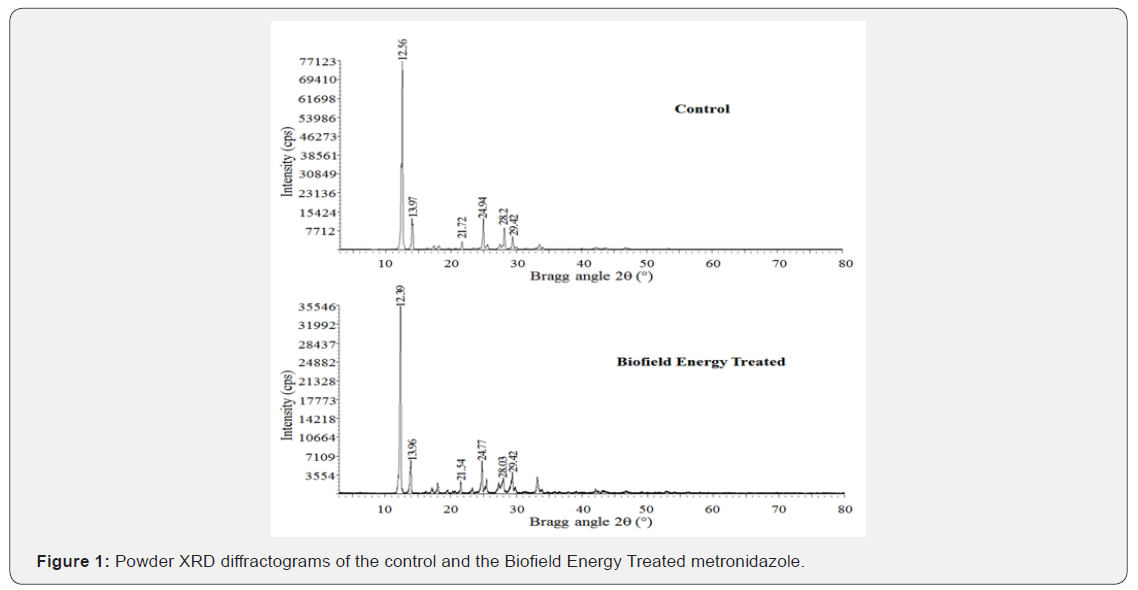
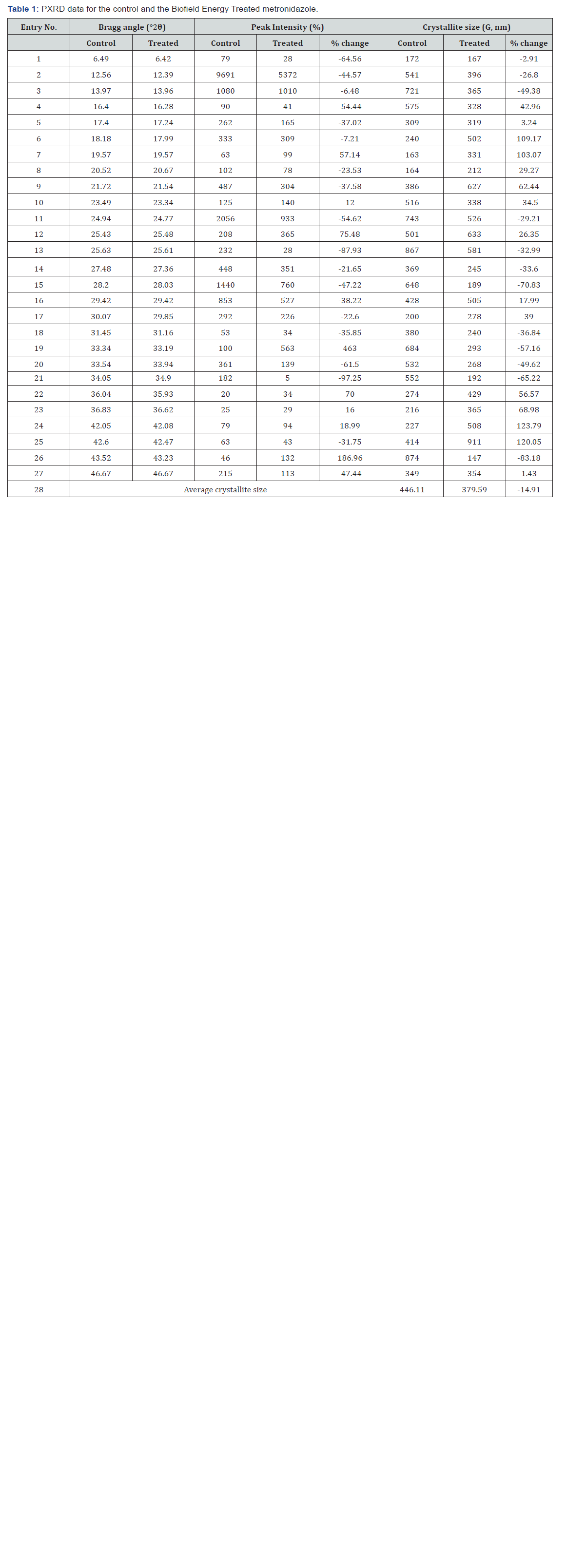
Similarly, the crystallite sizes of the Biofield Energy Treated metronidazole sample were significantly altered ranging from -83.18% to 123.79% compared to the control sample. Overall, the average crystallite size of the Biofield Energy Treated metronidazole (379.59 nm) was significantly decreased by 14.91% compared with the control sample (446.11 nm).
The variations found in the crystallite sizes and peak intensities indicated the modification of the crystal morphology of the Biofield Energy Treated metronidazole compared to the control sample. The peak intensity of each diffraction face on the crystalline compound changes according to the crystal morphology [41] and alterations in the PXRD pattern provide the proof of polymorphic transitions [42,43]. The Consciousness Energy Healing Treatment (the Trivedi Effect®) probably produced the new polymorphic form of metronidazole through the Biofield Energy via neutrino oscillations [18]. Different polymorphic forms of pharmaceuticals have the significant effects on the drug performance, such as bioavailability, therapeutic efficacy, and toxicity, because of their thermodynamic and physicochemical properties like melting point, energy, stability, and especially solubility, are different from the original form [44,45]. Thus, it can be assumed that the Consciousness Energy Healing Treated metronidazole would be better in designing pharmaceutical formulations containing metronidazole.
Particle Size Analysis (PSA)
The PSD analysis of both the control and the Biofield Energy Treated metronidazole were performed, and the data are presented in (Table 2). The particle size values of the control metronidazole at d10, d50, d90, and D (4,3) were 146.78 μm, 307.35 μm, 624.32 μm, and 356.11 μm, respectively. Similarly, the particle sizes of the Biofield Energy Treated metronidazole at d10, d50, d90, and D (4,3) were 125.21 μm, 274.09 μm, 504.82 μm, and 296.01 μm, respectively. Therefore, the particle size values in the Biofield Energy Treated metronidazole were significantly decreased at d10, d50, d90, and D (4,3) by 14.69%, 10.82%, 19.14%, and 16.88%, respectively compared to the control sample.
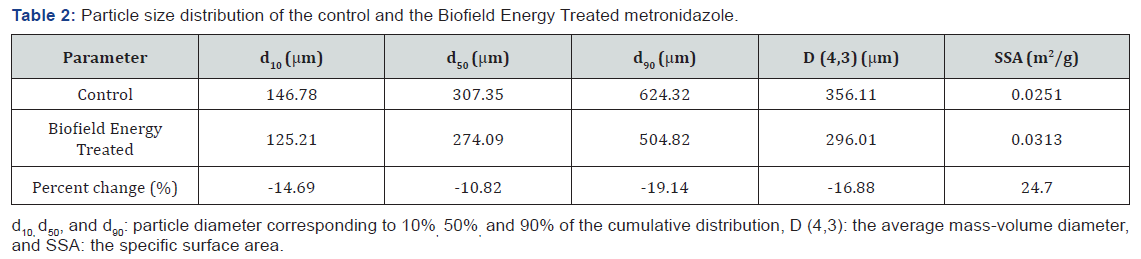
The specific surface area of the Biofield Energy Treated metronidazole (0.0251 m2/g) was significantly increased by 24.7% compared to the control sample (0.0313 m2/g). Hence, it can be assumed that the Trivedi Effect®-Consciousness Energy Healing Treatment might act as an external force for breaking the larger particles to smaller particles in size of metronidazole sample, hence increased the surface area. It was reported that the particle size, shape, and surface area have their impact on the solubility, dissolution rate, absorption, bioavailability, and even the therapeutic efficacy if it is a drug [45,46]. The solubility profile of metronidazole is very poor, where is very slightly soluble in water, alcohol, dilute acid, chloroform, and dimethylformamide [10,11]. Thus, it is anticipated that the Biofield Energy Treated metronidazole might show the enhanced therapeutic properties of pharmaceutical formulations and would be better for the industry using it as a raw material for the manufacturing.
Differential Scanning Calorimetry (DSC) Analysis
The DSC thermograms of both control and the treated metronidazole are presented in (Figure 2). The DSC thermograms of the control and the treated metronidazole showed the sharp endothermic peak at 162.01°C and 161.55°C, respectively (Figure 2). Similarly, the control and the Biofield Energy Treated samples showed exothermic peaks at 288.01°C and 287.55°C, respectively (Figure 2). The thermogram pattern and melting point closely matched to the literature reported data [10]. The melting point and decomposition temperature of the Biofield Energy Treated metronidazole were decreased by 0.28% and 0.16%, respectively compared with the control sample (Table 3). The melting and decomposition temperatures of the Biofield Energy Treated sample were decreased compared to the control sample. The melting point has been reported to decrease with decreasing particle size [47]. The particle size results justified the decreased thermal behavior of the Biofield Energy Treated metronidazole compared to the control sample.
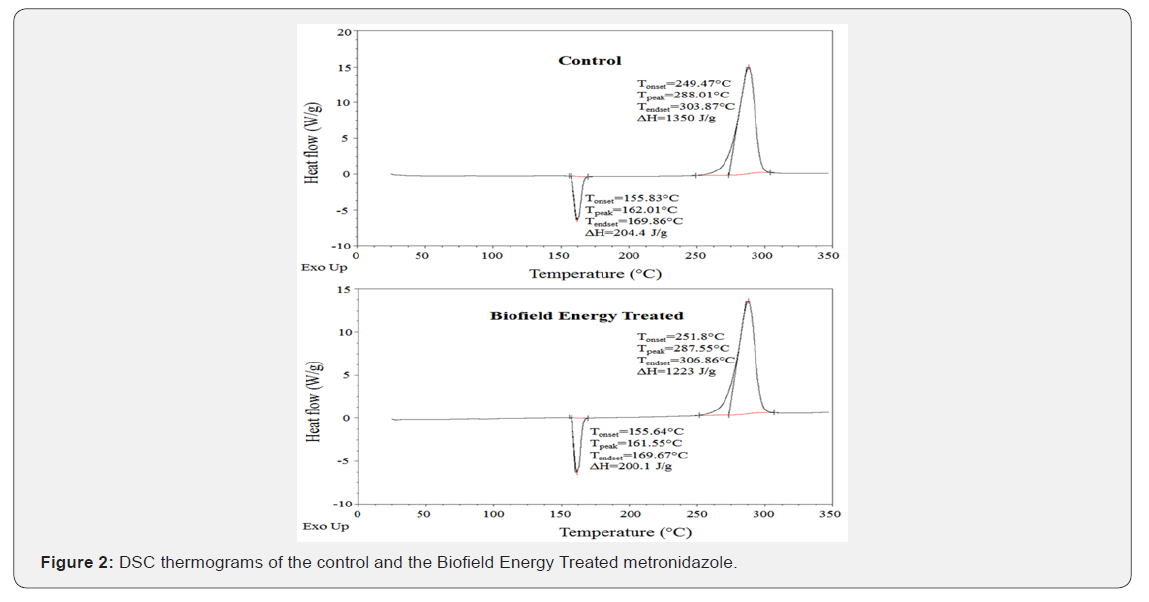
The latent heat of fusion (ΔHfusion) of the Biofield Energy Treated metronidazole (161.55 J/g) was decreased by 2.1% compared with the control sample (200.1 J/g) (Table 3). Similarly, the latent heat of decomposition (ΔHdecomposition) of the Biofield Energy Treated metronidazole (1223 J/g) was significantly decreased by 9.41% compared with the control sample (1350 J/g) (Table 3). The literature says that any change in the latent heat of fusion can be attributed to the disrupted molecular chains and the crystal structure [48]. Thus, it can be predicted that the Trivedi Effect ®-Consciousness Energy Healing Treatment might be responsible for the disruption the molecular chains and crystal structure of metronidazole which was the cause of declined thermal stability of the treated sample compared with the control sample.
Thermal Gravimetric Analysis (TGA) / Differential Thermogravimetric Analysis (DTG)
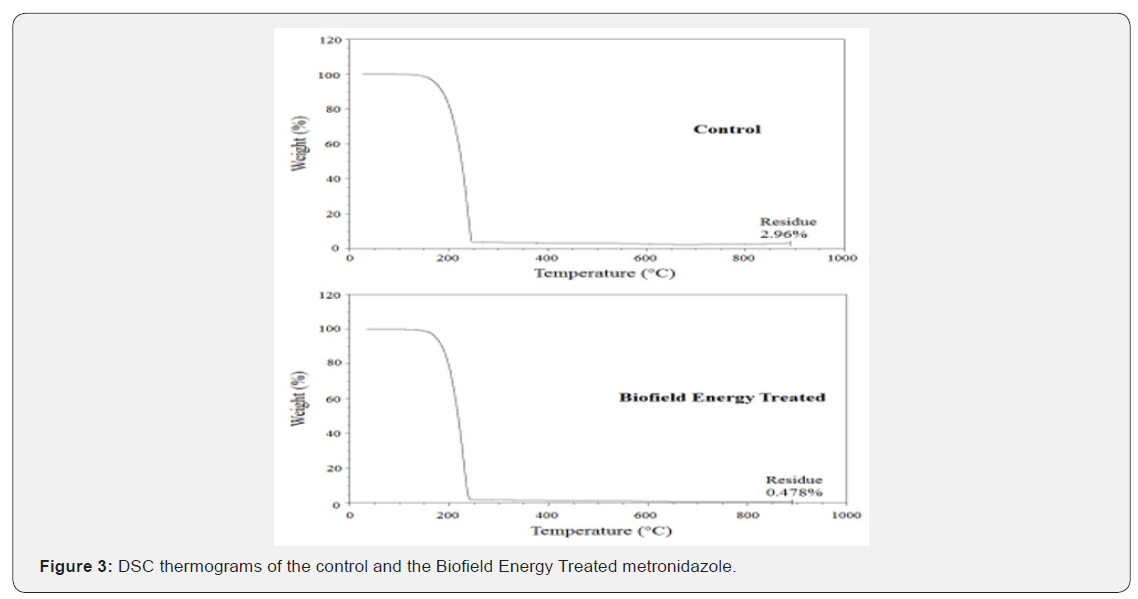
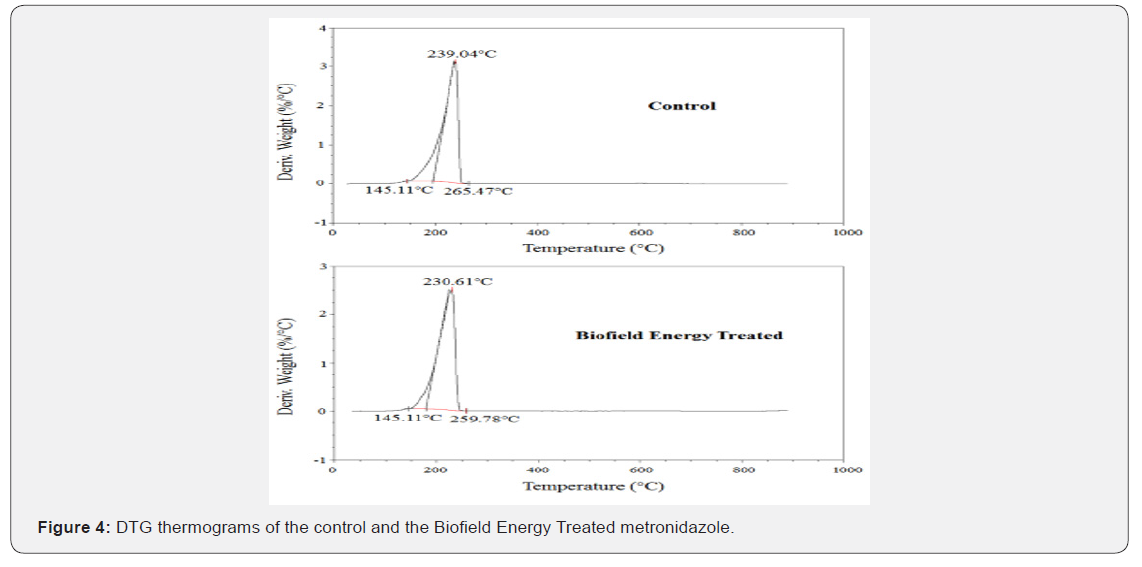
The TGA thermograms of the control and the Biofield Energy Treated metronidazole samples showed one step of thermal degradation (Figure 3). The Biofield Energy Treated metronidazole suffered a total weight loss of 2.56% more compared to the control sample (Table 4). Therefore, the residue amount was significantly decreased by 83.86% in the Biofield Energy Treated metronidazole compared to the control sample (Table 4). The DTG of the control and the Biofield Energy Treated metronidazole also showed one peak in the thermograms (Figure 4). The Tmax of the Biofield Energy Treated sample was decreased by 3.53% compared to the control sample (Table 4). Overall, TGA/DTG analysis of metronidazole samples revealed that the thermal stability of the Biofield Energy Treated sample was decreased compared with the control sample.
Conclusion
The experimental results showed that the Trivedi Effect® (Consciousness Energy Healing Treatment) has a significant effect on the particle size, surface area, and thermal properties of metronidazole. The peak intensities and crystallite sizes of the Biofield Energy Treated metronidazole were significantly altered ranging from -97.25% to 463% and -83.18% to 123.79%, respectively; however, the average crystallite size was significantly decreased by 14.91% compared with the control sample. The particle size values in the Biofield Energy Treated metronidazole were significantly decreased by 14.69%(d10), 10.82%(d50), 19.14%(d90), and 16.88% {D (4,3)}; thus, the specific surface area was significantly increased by 24.7% compared to the control sample. The latent heat of fusion and latent heat of decomposition were decreased by 2.1% and 9.41%, respectively in the Biofield Energy Treated sample compared with the control sample.
The total weight loss was increased by 2.56%; however, the residue amount was significantly decreased by 83.86% in the Biofield Energy Treated sample compared with the control sample. The maximum thermal degradation temperature was decreased by 3.53% in the treated sample compared with the control sample. From the results, it can be concluded that the Trivedi Effect®-Consciousness Energy Healing Treatment might have generated a new polymorphic form of metronidazole which may offer better solubility, dissolution, and good bioavailability compared with the control sample. The Trivedi Effect®-Consciousness Energy Healing Treated metronidazole would be very useful to design better pharmaceutical formulations that might offer better therapeutic response against bacterial and protozoal infections in the vagina (bacterial vaginosis), stomach (giardiasis, pseudomembranous colitis), liver, skin, joints (pelvic inflammatory disease), brain, and respiratory tract, aspiration pneumonia, rosacea, fungating wounds, intra-abdominal infections, lung abscess, periodontitis, amoebiasis, oral infections, and infections caused by susceptible anaerobic organisms such as Bacteroides, Clostridium, Fusobacterium, Dracunculus, Peptostreptococcus, Helicobacter pylori, and Prevotella species, etc.
Acknowledgement
The authors are grateful to Central Leather Research Institute, SIPRA Lab. Ltd., Trivedi Science, Trivedi Global, Inc., Trivedi Testimonials, and Trivedi Master Wellness for their assistance and support during this work.
References
- Metronidazole (2018) The American Society of Health-System Pharmacists.
- https://en.wikipedia.org/wiki/Metronidazole.
- Joesoef MR, Schmid GP, Hillier SL (1999) Bacterial vaginosis: Review of treatment options and potential clinical indications for therapy. Clin Infect Dis 28 Suppl 1: S57-65.
- Shennan A, Crawshaw S, Briley A, Hawken J, Seed P, et al. (2006) A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: The PREMET Study. BJOG 113(1): 65-74.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB (2007) A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 45(3): 302-307.
- Barr SC, Bowman DD, Heller RL (1994) Efficacy of fenbendazole against giardiasis in dogs. Am J Vet Res 55(7): 988-990.
- (2018) https://www.drugs.com/metronidazole.html.
- Kling PA, Burman LG (1989) Serum and tissue pharmacokinetics of intravenous metronidazole in surgical patients. Acta Chir Scand. 155(6-7): 347-350.
- Kling PA, Burman LG (1989) Serum and tissue pharmacokinetics of intravenous metronidazole in surgical patients. Acta Chir Scand 155(6- 7): 347-350.
- (2018) http://www.sciencelab.com/msds.php?msdsId=9925551.
- (2018) https://pubchem.ncbi.nlm.nih.gov/compound/ metronidazole#section=Solubility.
- Chereson R (2009) Bioavailability, bioequivalence, and drug selection. In: Makoid CM, Vuchetich PJ, Banakar UV (Eds) Basic pharmacokinetics (1st Edn) Pharmaceutical Press, London.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Sethi KK, et al. (2016) Isotopic abundance ratio analysis of biofield energy treated indole using gas chromatography-mass spectrometry. Science Journal of Chemistry 4(4): 41-48.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Panda P, et al. (2016) Evaluation of the isotopic abundance ratio in biofield energy treated resorcinol using gas chromatography-mass spectrometry technique. Pharm Anal Acta 7: 481.
- Branton A, Jana S (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. International Journal of Clinical and Developmental Anatomy 3(3): 9-15.
- Branton A, Jana S (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. American Journal of Clinical and Experimental Medicine 5(4): 138-144.
- Branton A, Jana S (2017) Effect of the biofield energy healing treatment on the pharmacokinetics of 25-hydroxyvitamin D3 [25(OH) D3] in rats after a single oral dose of vitamin D3. American Journal of Pharmacology and Phytotherapy 2(1): 11-18.
- Trivedi MK, Mohan TRR (2016) Biofield energy signals, energy transmission and neutrinos. American Journal of Modern Physics 5(6): 172-176.
- Rubik B, Muehsam D, Hammerschlag R, Jain S (2015) Biofield science and healing: history, terminology, and concepts. Glob Adv Health Med 4(Suppl): 8-14.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Koithan M (2009) Introducing complementary and alternative therapies. J Nurse Pract 5(1): 18-20.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, Latiyal O, Jana S (2015) Evaluation of atomic, physical, and thermal properties of bismuth oxide powder: An impact of biofield energy treatment. American Journal of Nano Research and Applications 3(6): 94-98.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. Ind Eng Manage 4: 161.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Physicochemical and spectroscopic characterization of biofield treated triphenyl phosphate. American Journal of Applied Chemistry 3(5): 168-173.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Impact of biofield treatment on spectroscopic and physicochemical properties of p-nitroaniline. Insights in Analytical Electrochemistry 1: 1-8.
- Mahendra KT, Rama MT, Alice B, Dahryn T, Gopal N, et al. (2015) Biofield treatment: A potential strategy for modification of physical and thermal properties of gluten hydrolysate and ipomoea macroelements. J Nutr Food Sci 5: 414.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Evaluation of biofield energy treatment on physical and thermal characteristics of selenium powder. Journal of Food and Nutrition Sciences 3: 223-228.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Bairwa K, et al. (2015) Spectroscopic characterization of disulfiram and nicotinic acid after biofield treatment. J Anal Bioanal Tech 6: 265.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Saikia G, et al. (2015) Physical and structural characterization of biofield treated imidazole derivatives. Nat Prod Chem Res 3: 187.
- Trivedi MK, Branton A, Trivedi D, Gangwar M, Jana S (2015) Antimicrobial susceptibility, biochemical characterization and molecular typing of biofield treated Klebsiella pneumoniae. J Health Med Inform 6: 206.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, Jana S (2015) Antibiogram, biochemical reactions, and genotypic pattern of biofield treated Pseudomonas aeruginosa. J Trop Dis 4: 181.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Bairwa K, et al. (2015) Effect of biofield treatment on physical, thermal, and spectral properties of SFRE 199-1 mammalian cell culture medium. Advances in Biochemistry 3: 77-85.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Bairwa K, et al. (2015) Physical, thermal, and spectroscopic characterization of biofield energy treated murashige and skoog plant cell culture media. Cell Biology 3: 50-57.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Jana S, et al. (2015) Evaluation of plant growth regulator, immunity and DNA fingerprinting of biofield energy treated mustard seeds (Brassica juncea). Agriculture, Forestry and Fisheries 4: 269-274.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Agronomic characteristics, growth analysis, and yield response of biofield treated mustard, cowpea, horse gram, and groundnuts. International Journal of Genetics and Genomics 3: 74-80.
- MiniFlex (1997) Desktop X-ray Diffractometer. The Rigaku Journal 14: 29-36.
- Zhang T, Paluch K, Scalabrino G, Frankish N, Healy AM, et al. (2015) Molecular structure studies of (1S,2S)-2-benzyl-2,3-dihydro-2- (1Hinden-2-yl)-1H-inden-1-ol. J Mol Struct 1083: 286-299.
- Langford JI, Wilson AJC (1978) Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J Appl Cryst 11: 102-113.
- Trivedi MK, Sethi KK, Panda P, Jana S (2017) A comprehensive physicochemical, thermal, and spectroscopic characterization of zinc (II) chloride using X‑ray diffraction, particle size distribution, differential scanning calorimetry, thermogravimetric analysis/ differential thermogravimetric analysis, ultraviolet-visible, and Fourier transform‑infrared spectroscopy. International Journal of Pharmaceutical Investigation 7(1): 33-40.
- Trivedi MK, Sethi KK, Panda P, Jana S (2017) Physicochemical, thermal and spectroscopic characterization of sodium selenate using XRD, PSD, DSC, TGA/DTG, UV-vis, and FT-IR. Marmara Pharmaceutical Journal 21/2: 311-318.
- Raza K, Kumar P, Ratan S, Malik R, Arora S (2014) Polymorphism: The phenomenon affecting the performance of drugs. SOJ Pharm Pharm Sci 1: 10.
- Brittain HG (2009) Polymorphism in pharmaceutical solids in Drugs and Pharmaceutical Sciences, volume 192, (2nd Edn), Informa Healthcare USA, Inc., New York.
- Censi R, Martino PD (2015) Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 20: 18759-18776.
- Blagden N, de Matas M, Gavan PT, York P (2007) Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev 59: 617-630.
- Chereson R (2009) Bioavailability, bioequivalence, and drug selection. In: Makoid CM, Vuchetich PJ, Banakar UV (eds) Basic pharmacokinetics (1st edn) Pharmaceutical Press, London.
- Khadka P, Ro J, Kim H, Kim I, Kim JT, Kim H, Cho JM, Yun G, Lee J (2014) Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci 9: 304-316.
- Antoniammal P, Arivuoli D (2012) Size and shape dependence on melting temperature of gallium nitride nanoparticles. Journal of Nanomaterials, Article ID 415797, 11.
- Zhao Z, Xie M, Li Y, Chen A, Li G, Zhang J, Hu H, Wang X, Li S (2015) Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. Int J Nanomedicine 10: 3171-3181.






























