Effect of Season on In VitroAnti-Oxidant Activity of SyzygiumCuminiL.Leaves
Prasenjit Mitra1, Prasanta Kumar Mitra2* and Tanaya Ghosh2
1Department of Biochemistry, All India Institute of Medical Sciences, India
2Department of Medical Biotechnology, Sikkim Manipal University, India
Submission: June 02, 2018;Published: July 03, 2018
*Corresponding author: Prasanta Kumar Mitra, Professor & Head, Department of Medical Biotechnology, SMIMS, SMU, India
How to cite this article: Prasenjit M, Prasanta K M, Tanaya G. Effect of Season on In Vitro Anti-Oxidant Activity of SyzygiumCuminiL.Leaves. Glob J Pharmaceu Sci. 2018; 5(5): 555673. DOI: 10.19080/GJPPS.2018.05.555673.
Abstract
Syzygiumcumini Linn (S. cuminiL.)is a medicinal plant. Anti oxidant activity of S. cuminiL. leaves is known in literature. In the present study we have examined effect of season on in vitroanti oxidant activity of S. cuminiL..leaves. Leaves ofS. cuminiL..were collected in different seasons and in vitroanti oxidant activity of the leaves was measured by superoxide anion generation with the help of linoleic acid peroxidation assay, xanthine-xanthine oxidase assay and by DPPH photometric assay. Anti oxidant compounds like total phenol, ascorbic acid, flavonoids and carotenoid present in the leaves of different seasons were also estimated. Results showed that in vitroanti oxidant activity of S. cuminiL..leaves was maximum during summer (March-May). Anti oxidant activity was related with high content of total phenol, ascorbic acid, flavonoid and carotenoids in the leaves. It is therefore concluded that leaves ofS. cuminiL..of summer should be used to get maximum anti oxidant activity.
Keywords: Syzygiumcuminileaves; Anti-oxidant activity; Total phenol; Flavonoid; Ascorbic acid; Carotenoids; Effect of season
Introduction
Numerous medicinal plants are known possessing anti oxidant activity. Few of them are, Piper longum L., Solanum nigrum L., Amaranthus caudatus L., Desmodium gangeticum L., Ocimum sanctum L., Eclipta alba L, Hyptis suaveolen L., Alpina calcarata L. , Ocimum basillicum L., Jatropa multifida L. , Hyptis suaveolens L., Solanum indicum L. , Clitorria ternate L. etc. [1,2]. Syzygium cumini L. (family Myrtaceae) is a tropical fruit tree of great economic importance. It is a large evergreen tree up to 30m height and a girth of 3.6m with a bole up to 15m. The plant is native to Nepal, Pakistan, Bangladesh, India, and Indonesia. In India the plant is found almost everywhere. In English the plant is known as Jambul tree. In Hindi, Bengali, Punjabi, Tamil, Gujrati and Malayalam the plant is called as Jamuna, Jaam, Jammun, Naval, Gambu and Njaval respectively [3].
S. cumini L. is known to possess a wide range of medicinal properties. Leaf has anti diabetic, anti allergic, anti viral, anti bacterial, anti DNA damage and anti oxidant activities. Fruit is anti-hyper lipidemic, possessing anti-cancer property. Seeds exert anti inflammatory and anti gastric ulcer activity. Bark and pulp of the plant are efficacious for diabetes [4].
Phytochemical studies showed that stem bark of S. cumini L. contains betulinic acid, ß-sitosterol, ß-sitosterol-D-glucoside, quercetin, myricetin, astragalin kaempferol-3-o glucoside, friedelin, epi-friedelanol, eugenin and gallic acid. Leaves contain n-hepatcosane, n-nonacosane, sitosterol,betulinic acid, crategolic (maslinic) acid, acid soxalic, citric acid, glycolic acids, n-hentriacontane, n-octacosanol, n-triacontanol, kaempferol 3-0-ß-D-glucuronopyranoside, ellagitannin, nilocitin, myricetin 3-0-ß-D-glucaronopyranoside and aminoacids like glycine, alanine etc. Oleanolic acid, erategolic acid (maslinic acid), quercetin, kaempferol and myricetin flavonoids -isoquercitrin were found in the flowers of S. cumini [5,6].
Anti oxidant property of S. cumini L. leaf is known in literature. Using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical-scavenging and ferric-reducing antioxidant power (FRAP) assays Ruan et al. showed that that water, ethyl acetate, chloroform, n-hexane and methanol extracts of S. cumini L. leaf have anti oxidant activity [7]. By using the same methods Eshwarappa et al. also showed antioxidant activity of S. cumini leaf gall extracts [8]. In the present study effect of season on in vitro anti oxidant property of S. cumini L. leaf was investigated.
Methodology
Plant material
S. cumini L. leaves were collected from the medicinal plants garden of the University of North Bengal, Dist. Darjeeling, West Bengal, India during Autumn (September-November), Winter (December-February), Summer (March-May) and rainy season (June-August) at about 9 AM. Leaves were authenticated by the experts of the department of Botany of the said university. A voucher specimen was kept in the department of Medical Biotechnology, Sikkim Manipal Institute of Medical Sciences of the Sikkim Manipal University, Gangtok, Sikkim, and India for future references.
Test material
Collected leaves of S. cumini L. were shade dried and powdered. The powder was used as test material
Chemicals
Chemicals required for the study were purchased from Himedia Lab, Loba Chem. Lab, India and from Merck, Germany.
Antioxidant assays
Antioxidant activity of S. cumini L. leaves of different seasons was assayed by superoxide anion generation by xanthine-/ xanthine oxidase assay [9], linoleic acid peroxidation assay [10] and by DPPH photometric assay [11].
Flavonoids content
Flavonoids content of S. cumini L. leaves of different seasons was determined using Aluminum chloride colorimetric method [12].
Total phenols content
Total phenols content of S. cumini L. leaves of different seasons was determined by Folin Ciocalteu reagent [13].
Ascorbic acid content
Ascorbic acid content of S. cumini L. leaves of different seasons was determined by the method of Cakmak and Marschner [14].
Carotenoids content
Total carotenoids of S. cumini L. leaves of different seasons was determined by the method of Jensen [15].
Statistical Analysis
The statistical significance between antioxidant activity values of the extracts was evaluated with a Duncan’s multiple range test (DMRT) at 5% were considered to be statistically significant [16].
Results and Findings
Effect of seasons on in vitro antioxidant activity of powdered leaves of S. cumini L. through superoxide anion generation by linoleic acid peroxidation assay, xanthine-/xanthine oxidase assay and by DPPH photometric assay is tabulated in Table 1.
Concentration used: 100μg/ml Dose was fixed based on our earlier report [17]. Results were a mean of triplicate experiments ±SEM. In vitro anti oxidant activity of the powdered leaves of S. cumini L. was notices in all seasons but maximum activity was found during summer (March-May). Inhibitions in xanthine oxidase, linoleic acid peroxidation and DPPH were found 91%, 78% and 90% respectively. Results were comparable to that of quercetin, a known anti-oxidant compound, where inhibition in linoleic acid peroxidation came 87% but for both xanthine oxidase and DPPH inhibition was 100% (Table 1). Results were a mean of triplicate experiments ±SE.
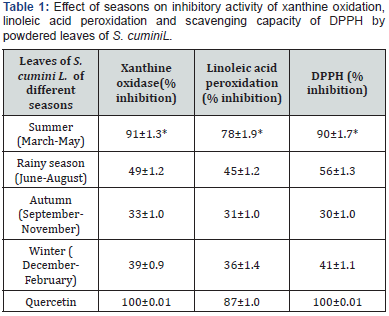
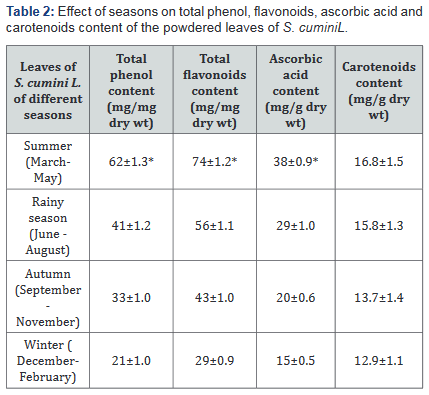
Table 2 shows that total phenol content of the leaves of S. cumini L. in summer was 62±1.3mg/mg dry wt of the leaves. This was maximum when compared to total phenol content of the leaves in other seasons of the year. During autumn, winter and rainy seasons total phenol content of S. cumini L. leaves were 33±1.0, 21±1.0 and 41±1.2mg/mg dry wt respectively. Same trend was found in total flavonoids, ascorbic acid content of S. cumini L. leaves. Carotenoids content in S. cumini L. leaves, however, did not show any significant change in different seasons of the year.
Discussion
Fluck and Pharm in the year 1955 showed influence of climate on the active principles in medicinal plants. Thereafter, researchers conducted experiments in this direction and demonstrated influence of season on concentration of active compounds in plants [18-22]. We have also undertaken experiments on seasonal variation in concentrations of active metabolites in plants and noted that season can change amount of bio active compounds in different parts of the plants [23-27](Figure 1).

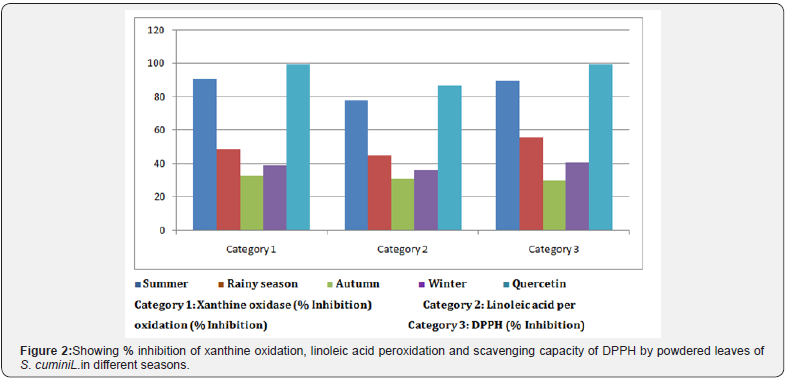
In present study effect of season on in vitro anti oxidant activity of S. cumini L. leaves was studied. Results showed that anti oxidant activity of S. cumini L. leaves in terms of inhibitions in linoleic acid peroxidation, xanthine oxidase and DPPH was maximum during summer season (March-May) and the anti oxidant activity was comparable to that of synthetic anti-oxidant quercetin (Figure 2). Aysel and Sevcan in 2014 showed that antioxidant activity of Prunus amygdalus L. reached the highest value in April for leaves whereas in October for stems [28]. Bahmanzadegan et al. in 2015 demonstrated the best antioxidant activity of Laurus nobilis L. was in spring and the lowest one was in winter [29].
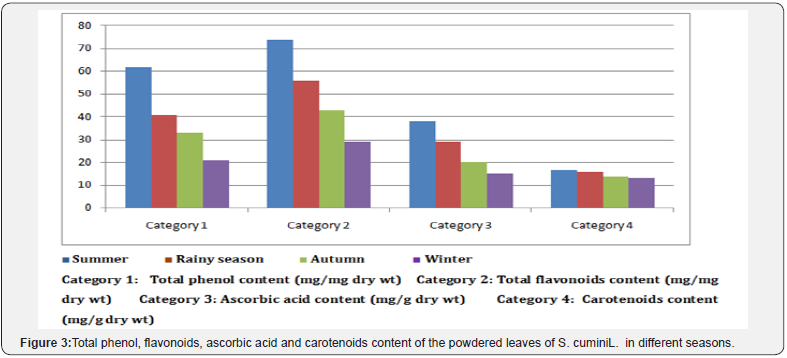
Miller in 1996 found that anti oxidant activity of medicinal plant is mainly due to presence of flavonoids, ascorbic acid, phenolic compounds and carotenoids. These chemicals are responsible for multiple biological effects like free radical scavenging abilities, anti inflammatory and anti carcinogenic activities [30]. We, therefore, studied effect of season on these anti oxidant compounds in S. cumini L. leaves. Results (Figure 3) showed that amounts of phenolic compounds, ascorbic acid and flavonoids in the plant leave were maximum during summer (March-May). In 2014 Aysel and Sevcan found that highest level of total phenolic compounds in Prunus amygdalus L. was in January for stems while in October for leaves [28]. In vitro anti oxidant activity of S. cumini L. leaves during summer was, therefore, due to accumulation of maximum amount of phenolic compounds, ascorbic acid and flavonoids in the plant leaves.
Oxidative process develops free radicals in living systems. These free radicals are major causative factors for induction of many chronic and degenerative diseases including diabetes mellitus, cancer, atherosclerosis, ischemic heart disease, neurodegenerative diseases, ageing; immunosuppressant etc. [31]. Ani oxidants exert protective effects against oxidative stress in biological systems [32]. Therefore, search of anti oxidants are of utmost importance to combat oxidative stress and the related diseases.
Synthetic anti-oxidants like butylated hydroxyanisole and butylated hydroxytoluene are commercially available. They are commonly used in processed food also. But, their toxicity is a matter of concern. It is often claimed that these synthetic anti oxidants have many side effect including carcinogenic activity [33]. Therefore, there are high demands for naturally occurring anti oxidants. As the present study indicates that March-May (Summer season) is the period when S. cumini L. leaves showed maximum in vitro anti oxidant activity, S. cumini L. leaves of summer may be used as the source of natural anti oxidant.
Conclusion
Effect of season on in vitro anti oxidant activity of S. cumini L. leaves was studied. Results showed that in vitro anti oxidant activity of S. cumini L. leaves was maximum during summer season (March-May). The anti oxidant effect was due to presence of maximum amount of phenols, flavonoids and ascorbic acid in the leaves during summer.
Recommendation
S. cumini L. leaves of summer season may be used as the source of natural antioxidant.
References
- Prakash V, Mishra PK, Mishra M (2009) Screening of medicinal plant extracts for antioxidant activity. Journal of Medicinal Plants Research 3(8): 608-612.
- Narayanaswamy N, Balakrishnan KP (2011) Evaluation of some Medicinal Plants for their Antioxidant Properties. International Journal of Pharm Tech Research 3(1): 381-385.
- Jadhav VM, Kamble SS, Kadam VJ (2009) Herbal medicine: Syzygium cumini: A Review. Journal of Pharmacy Research 2(8): 1212-1219.
- Chaudhary B, Mukhopadhyay K (2012) Syzygium cumini (L.) Skeels: A potential source of nutraceuticals. International Journal of Pharmacy and Biological Sciences 2(1): 46-53.
- Bhargava KK, Dayar R, Seshadri TR (1974) Chemical components of Eugenia jambolana stem bark. Current Sci 43(20): 645- 646.
- Gupta GS, Sharma DP (1974) Triterpenoid and other constituents of Eugenia jambolana leaves. Phytochemistry 13: 2013-2014.
- Ruan ZP, Zhang L, Lin-Yi M (2008) Evaluation of the Antioxidant Activity of Syzygium cumini Leaves. Molecules 13(10): 2545-2556.
- Eshwarappa RSB, Iyer RS, Subbaramaiah SR, Austin RS, Dhananjaya BL (2014) Antioxidant activity of Syzygium cumini leaf gall extracts. BioImpacts 4(2): 101-107.
- Chang WS, Chang YH, Lu FJ, Chiang HC (1994) Inhibitory effects of phenolics on xanthine oxidase. Anticancer Res 14(2A): 501-506.
- Chang W, Choi, Sei C, Kim, Soon S, et al. (2002) Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay- guided comparison. Plant Science 163(6): 1161- 1168.
- Mensor LL, FMenezes FS, GLeita GG, Reis AS, Dos Santos TC, et al. (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15(2): 127-130.
- Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content inpropolis by two complementary colorimetric methods. J Food Drug Analaysis 10(3): 178-182.
- McDonald S, Prenzler PD, Autolovich M, Robards K (2001) Phenolic content and antioxidant activity of olive extracts. Food Chemistry 73(1): 73-84.
- Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol 98(4): 1222-1227.
- Jensen A (1978) Chlorophyll and carotenoids. In: Hallebust JA, Craigie JS (Eds), Handbook of Physiochemical and Biochemical Methods. Cambridge University Press, Cambridge, UK, England, pp. 5-70.
- Bliss CI (1967) Statistics in biology, Statistical methods for research in the natural sciences, Vol. 1. McGraw Hill Book Company, NY, USA, pp. 558.
- Mitra PK, Ghosh T, Mitra P (2016) In vitro antioxidant activity of chromatographically separated fractions from the leaves of Aastilbe rivularis buch. – Ham. Ex D. Don. SMU Medical Journal 3(2): 226-239.
- Fluck H, Pharm M (1955) The influence of climate on the active principles in medicinal plants. J Pharm Pharmacol 7(1): 361-383.
- Gupta PL (1977) Variation in morphological characters and active principle constituents of E. prostrata Linn. under different seasonal and soil conditions. JRIM 12(1): 80-84.
- Schultz JC, Nothnagle PJ, Baldwin IT (1982) Seasonal and individual variation in leaf quality of two northern hardwoods tree species. American Journal of Botany 69(5): 753-759.
- Mauffette Y, Oechel WC (1989) Seasonal variation in leaf chemistry of the coast liveoak Quercus agrifolia and implications for the California oak moth. Phryganidia californica Oecologia 79(4): 439-445.
- Bahmanzadegan A, Rowshan V, Zareian F, Alizadeh R, Bahmanzadegan M (2015) Seasonal Variation in Volatile Oil, Polyphenol Content and Antioxidan Activity in Extract of Laurus nobilis Grown in Iran. Journal of Pharmacy and Pharmacology 3: 223-231.
- Mitra PK (2014) In vitro antibacterial activity of leaves of Amaranthus spinosus L: Seasonal variation. World Journal of Pharmaceutical Sciences 2(12): 1702-1706.
- Ghosh T, Mitra P, Dilip JK, Mitra PK (2015) A study on body weight loss in rats by the leaves of Abrus precatorius Linnaeus: Effect of season. International Journal of Pharmacy & Therapeutics 6(Suppl 1): 64-68.
- Mitra P, Ghosh T, Mitra PK (2016) Seasonal variation in antiulcerogenic activity of ageratum conyzoides l leaves. International Journal of Biopharmaceutics 7(2): 63-68.
- Mitra PK, Ghosh T, Mitra P (2016) In vitro anti thiamine effect of murrya koenigii (linn.) spreng wettst leaves: effect of season. International Journal of Biopharmaceutics 7(2): 69-72.
- Mitra P, Ghosh T, Mitra Prasanta K (2017) Effect of Season on In vitro Anti-Oxidant Activity of Aageratum conyzoides L. Leaves. SMU Medical Journal 4(2): 222-233.
- Sivaci A, Duman S (2014) Evaluation of seasonal antioxidant activity and total phenolic compounds in stems and leaves of some almond (Prunus amygdalus L.) varieties Biological Research 47(9): 1-5.
- Bahmanzadegan A, Rowshan V, Zareian F, Alizadeh R, Bahmanzadegan M (2015) Seasonal Variation in Volatile Oil, Polyphenol Content and Antioxidan Activity in Extract of Laurus nobilis Grown in Iran. Journal of Pharmacy and Pharmacology 3: 223-231.
- Miller AL (1996) Antioxidant flavonoids: structure, function and clinical usage. Alt Med Rev 1: 103-110.
- Young IS, Woodside JV (2001) Antioxidants in health and disease. J Clin Pathol 54(3): 176-186.
- Cao G, Sofic ER, Prior RL (1996) Antioxidant capacity of tea and common vegetables. J Agric Food Chem 44: 3426-3431.
- Branen AL (1975) Synthetic anti oxidants. Journal of American Oil Chemist Society 52: 59-63.






























