Peg-5 Oleate Based Self Microemulsifying Drug Delivery System: As a Versatile Approach in Oral Bioavailability Enhancement of Anti-Diabetic Agent: Formulation Design, vitro/In vivo Evaluation & Stability Studies
Vikas Pandey1 and Seema Kohli2*
1Department of Pharmaceutics, School of Pharmacy, Suresh Gyan Vihar University, India
2Departmen of Pharmaceutical Sciences, Kalaniketan Polytechnic College, India
Submission: August 02, 2017;Published: May 18, 2018
*Corresponding author: Seema Kohli, Dept of Pharmaceutical Sciences, Kalaniketan Polytechnic College, India
How to cite this article: Vikas P, Seema K. Peg-5 Oleate Based Self Microemulsifying Drug Delivery System: As a Versatile Approach in Oral Bioavailability Enhancement of Anti-Diabetic Agent: Formulation Design, vitro/In vivo Evaluation & Stability Studies. Glob J Pharmaceu Sci. 2018; 5(5): 555671. DOI: 10.19080/GJPPS.2018.05.555671.
Abstract
The rationale of this research work was to develop and characterize self-micro emulsifying drug delivery system (SMEDDS) of repaglinide (RPG). The solubility of RPG was analyzed in various excipients. Pseudo ternary phase diagram was used to evaluate the micro-emulsification existence area. SMEDDS formulation (ME1, ME2, ME3, ME4&ME5) were examined for micro-emulsifying properties, and the consequential formulations loaded with RPG were investigated for clarity, phase separation, globule size and shape, zeta potential, thermodynamic and thermal stability. Out of five formulations developed three (ME1 , ME2&ME3) were found to be suitable from the point of view of above mentioned parametrical studies and were consecutively subjected to stability studies as per International conference on Harmonization (ICH) guidelines. Accelerated and long term conditions of a period of 3 months indicated no such noteworthy changes. TEM micrographs of microemulsion formulations further assured the spherical shape of globules. Developed SMEDDS formulation showed superior performance in in-vitro drug release and in-vivo studies in contrast to marketed RPG formulation (tablets) and pure RPG drug.
Keywords:Lipid formulation; Medium chain triglycerides; Repaglinide; Particle size; Selfmicroemulsifying drug delivery system; Solubility; Bioavailability
Abbreviations: ICH: International Conference on Harmonization; GIT: Gastro-Intestinal Tract; TEM: Transmission Electron Microscopy
Introduction
The pervasiveness of diabetes among all age groups worldwide is expected to be 4.4% in 2030. Projections in mounting of total number of people globally is estimated to be from 171 million in 2000 to 366 million in 2030, urban population in developing countries is projected to double between 2010 and 2030 [1]. To clash with such a perilous disease several therapeutic molecules had been explored out of which one is RPG (2-ethoxy-4-[[3-methyl-1-[2-(1-piperidyl) phenyl]-butyl] carbamoylmethyl] benzoic acid) which is an effective second generation oral hypoglycemic agent extensively utilized in the treatment of non insulin dependent diabetes mellitus, which acts by blocking ATP-dependent potassium channels in pancreatic beta cells, which in turn, stimulates insulin secretion. RPG acts in a dose-dependent manner, and is characterized by a fast onset, yet short duration of action [2]. But still the battle does not end here since every potential candidate needs a sound formulation system for a successful onset of action and therapeutic effectiveness because of some associated issues like solubility, RPG has very low aqueous solubility (34μg/mL at 37 °C) and high lipophilicity (logP=3.97) hence if solubility issue of such a potential candidate can be taken away then definitely there would be fair chance of getting greater therapeutic effects [3].
In a pursuit of elimination of solubility issues several formulation strategies had been developed among which lipid based system had gained immense popularity over past years because of their ability to enhance the drug water-solubility and permeation, serving drug pass into the lymphatic vessels, and minimizing liver metabolism. Bypassing the dissolution process in the gastro-intestinal tract (GIT) by delivering the entire dose in solution thereby is considered to be one of the most important qualifications of this system [4], overall lipid-based drug delivery systems have been developed to overcome the possible adverse influence of P-glycoprotein [5]. Among various lipid based formulations self micro emulsifying based drug delivery system (SMEDDS) had shown substantial improved performance in resolving solubility issues. SMEDDS are isotropic and thermodynamically stable solutions comprising oil, surfactant, co surfactant and drug that instinctively form oil in water microemulsion when mixed with water under gentle agitation which is provided by digestive motility of stomach and intestine in vivo [6]. Due to smaller droplet size SMEDDS proffers larger interfacial surface area resulting in enhanced release and absorption properties [7,8]. In this study, SMEDDS consisting RPG were formulated with the intention of enhancing solubility and minimizing disparities in the bioavailability. The optimized SMEDDS formulations characterized for various physicochemical parameters (like droplets size and size distribution, zeta potential, dilution studies, thermodynamic stability studies morphology and thermal stability studies).
Materials and Methods
Materials
RPG was a generous gift sample from Torrent Pharmaceuticals Pvt. Ltd (Ahmedabad, India). Labrafac (caprylic/capric triglyceride), Labrafil (PEG-5 Oleate), Labrasol (Caprylocaproyl macrogol-8 glyceride), Capryol 90 (Propylene glycol monocaprylate), Transcutol (Diethylene glycol monoethyl ether), (Maisine) glyceryl monolinoleate, plurol oleique CC 497 (Polyglyceryl-3 oleate) were provided by Gattefosse Pvt. Ltd (Mumbai, India). Estol (Propylene glycol dicaprylate/caprate) was supplied by Croda Pvt. Ltd (Mumbai, India). Capmul MCM was provided by Abitec Corporation (USA). Chremophor RH40 was provided by BASF (Mumbai, India). Castor oil, olive oil, soya bean oil, Tween 80, PEG 400, ethanol, glycerol, glycerine and propylene glycol were purchased from (Sigma Aldrich Pvt. Ltd, New Delhi, India). Isopropyl myristate (IPM) was obtained from Loba chem. (Mumbai, India). All other chemicals and solvents were of analytical grade. Double distilled water was prepared freshly whenever required.
Methods
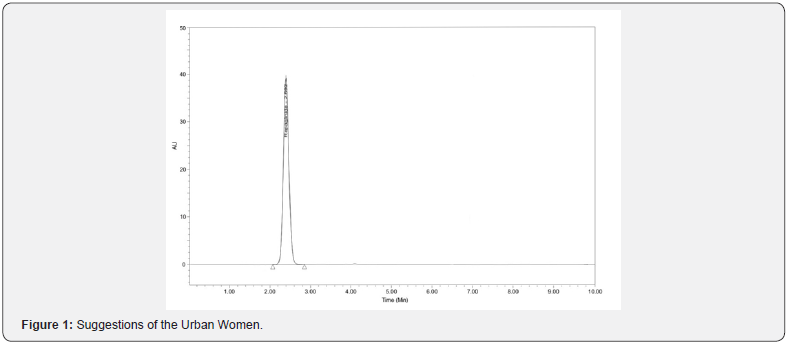
HPLC analysis of repaglinide: The concentration of RPG was determined by HPLC method. The system consists of Agilent 1220c infinity 2012 series with a manual injector, binary pump, UV detector and chemstation software. The chromatographic column was TC-C18 (250cm and 4.6mm i.d.) with 5μm particle size. The mobile phase (filtered through a 0.45μm membrane filter, degassed by ultrasonication for 15min) 70:30v/v was methanol and ammonium phosphate buffer (pH 2.5). Injection volume was 20μl and run time was 10min and flow rate 1.0ml/ min. The column was maintained at ambient temperature and the eluent was detected at 242nm. The retention time of RPG was 2.897min at ambient room temperature (Figure 1). The calibration curve was plotted as concentration of the drug versus the response at each level. The proposed method was evaluated by its correlation coefficient and intercept value calculated in the statistical study. They were represented by the linear regression equation Y = -15.743+1031.19X, ‘r’ value= 0.9991. Slopes and intercepts were obtained by using regression equation (y=mx+c) and least square treatment of the results used to confirm linearity of the method developed. The method was validated for accuracy, precision, specificity and solution stability.
Solubility studies of RPG in various excipients: The solubility of RPG in various lipids, surfactants and co surfactants was verified. An excess amount of RPG was introduced to 2mL of each excipient and the mixture in a capped cuvette was stirred in a water-bath at 25 °C for 48h; a vortex mixer was used to ease the solubilization if necessary. After standing for 24h and reaching equilibrium at ambient temperature, each cuvette was centrifuged at 3000rev min-1 for 10min using a centrifuge (Remi PR-23, India). Undisclosed RPG was removed by filtering with a membrane filter (0.45μm). The concentration of RPG was determined by the above-mentioned HPLC analysis.
Construction of pseudo-ternary phase diagrams: Pseudo-ternary phase diagrams were constructed using the water titration method consisting lipid, surfactant and co surfactant. Surfactant selected were Cremophor RH40, Tween 80, Labrasol, pleural oleique CC 497 and glycerine, pooled with cosurfactants as solubilizer namely (ethanol, propylene glycol, PEG 400 and glycerol). Lipids used were Labrafac, Labrafil, Estol, Maisine, Castor oil, olive oil, soyabean oil, isopropyl myristate (IPM, Capryol 90 and capmul MCM 18. A blend of surfactant with co surfactant were made having different ratios of 1:2, 1:1, 2:1, 3:1 (i.e. Km, w/w) followed by blending of Smix (volumes of each surfactant and corsurfactant) with lipid in a ratio of 9:1 to 1:9 (w/w), then water was added in a drop-wise manner to each lipid- Smix mixture under moderate shaking at 37 °C. Observation and evaluation of appearance, dispersibility, droplet size/distribution and zeta potential were recorded. A clear distinction was notified between the microemulsion which was clear and slight blue and the crude emulsion which had a milky white appearance. The quantity of water, lipid, surfactant and cosurfactant added was noted down and calculated. The pseudo-ternary phase diagrams were constructed using sigma plot V.10 software according to the data. The microemulsion regions in the diagrams were plotted and the broader area showed the superior self-microemulsification efficiency.
Preparation of RPG loaded liquid SMEDDS: After performing comparison of plotted phase diagrams, optimal combination of surfactant, co-surfactant and lipid were selected. SMEDDS formulations were further prepared by dissolving RPG into cosurfactant or Smix in a glass cuvette, heating at 37 °C in a water-bath or using a vortex mixer (Remi CM-101 PLUS, India) to facilitate the solubilization if necessary. Further this mixture was added to the calculated quantity of lipid into cuvette with continuous mixing until the homogenous mixtures had been formed. Ultimately, the mixture was kept at 25 °C. Formulations of SMEDDS of RPG were coded as (ME1, ME2, ME3, ME4, ME5 and ME6) and were subjected to further characterizations. Detailed compositions of SMEDDS formulations were presented in Table 1.
Preliminary in-vitro evaluation of selfmicroemulsification efficiency : Visual examination of SMEDDS concentration was performed after diluting with the medium, in which SMEDDS concentration of 0.2ml was taken in a volumetric flask and 20ml of 0.1 M HCL was added to it in a drop wise manner, temp kept was 37 °C. Dispersibility, appearance, flow ability and time of self emulsification was observed and recorded as per the five grading system presented in Table 2 [9- 12]. Effect of pH on self-micro emulsification competence was evaluated by taking 0.1 M HCL as a diluting medium.
Characterization of SMEDDS
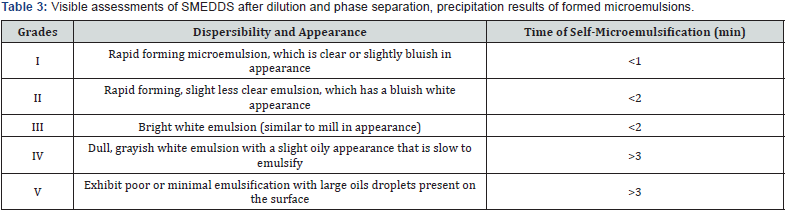
Visual observation, phase separation of emulsion: Each formulation of SMEDDS containing RPG was diluted with 200mL of distilled water at 37 °C to check visual appearance, the diluted preparation was vortexes for 1min, and then the mixtures was stored for a period of 24hrs, and phase separation and precipitation observed visually [10]. Mixtures exhibiting a negligible phase separation were used for subsequent studies.
Determination of self-emulsification time and optical clarity: The efficiency of self-micro emulsification was estimated by using magnetic stirrer at 100rpm, water and 0.1 NHCl solutions as medium [10-12]. Temperature was maintained at 37±0.5 °C. SMEDDS formulation was added into the medium and the contents being mixed gently at 100rpm and determining the time required to form microemulsion upon dilution of SMEDDS with water. Each formulation (1mL) was diluted with 100mL of water in glass spectrophotometer (Labtronics LT290, India) immediately after SMEDDS formation and after 0hr and 24hr respectively to determine optical clarity.
Droplet size analysis and zeta potential: The droplet size, size distribution and zeta potential were examined by dynamic light scattering with particle size apparatus (Malvern Zetasizer). Formulations were diluted with deionized water and 0.1 N HCl in a drop-wise manner at 25 °C under gentle shaking [13].
Cloud point measurement: This study provides information about stability of formulation system at body temperature. In this formulation was diluted with 50mL of water in beaker and placed on a water bath while gradually increasing the temperature until the diluted formulation turns cloudy [14].
Thermodynamic stability studies of RPG SMEDDS: The formulations were subjected to heating cooling cycle (4 °C and 45 °C) and freeze thaw cycle (-21 °C and +25 °C) with storage at each temperature of not less than 48h. For centrifugation stress, the formulations were centrifuged at 3500rpm for 15min and the extent of phase separation was monitored.
Drug release studies: Drug release experiments were conducted using a modified dialysis method [15]. At first, the dialysis tubing was soaked in the dialysis medium for 12h at room temperature which was treated at 40 °C before initiation of experiment. The diluted SMEDDS formulation (equivalent to 10mg) was placed in dialysis tubing and clamped on both sides. The secured dialysis tube was allowed to rotate freely in the dissolution vessel of USP type II dissolution apparatus (MODEL TDT-06T) (Electro lab, Mumbai, India) containing 900ml of simulated gastric fluid (SGF, pH 1.2 without enzyme) and simulated intestinal fluid (SIF, pH 6.8 without enzyme) as dialysis medium at 37±0.5 °C and stirred at 50rpm. RPG SMEDDS, conventional tablet (Eurepa, Torrent pharmaceuticals ltd, Repaglinide 2mg) were used for dissolution studies; results were compared with that of pure RPG. Samples were withdrawn at 5, 10, 20, 30, 45 and 60 min and filtered through 0.45μm membrane. An equal volume of fresh dissolution medium was concurrently replenished to maintain the volume constant. The amount of RPG dissolved in the dissolution medium was examined by HPLC analysis method as mentioned above. Results are averaged from three replicated experiments.
Transmission electron microscopy
From the results of thermodynamic stability studies formulations (ME1, ME2 & ME3) were selected for morphological characterization using transmission electron microscopy (TEM). Transmission electron microscope (TEM; JEOL USA JEM-2100F) was used as a visualizing aid. SMEDDS formulations were diluted with water (1:100). A drop of the diluted microemulsionwas directly deposited on the holey film grid and observed the morphology of formulations
Stability studies: Formulations, which were found to be thermodynamically stable, were subjected to stability studies. Samples of stability studies were charged on 25 °C±2 °C/60±5% RH (Newtronics NLHC21SI stability chamber, Newtronics, India) and 40 °C±2 °C/75±5% RH (Newtronics NLHC21SI stability chamber, Newtronics, India) Samples were subjected to stability studies for 3 months period.
In vivo evaluation
Experimental diabetes onset and blood glucose level measurement
In vivo studies were conducted after acquiring approval from Institutional Animal Ethics Committee, Bhopal, Madhya Pradesh (Registration No. Reg. No. 1411/PO/a/CPCSEA) and standardized guidelines of Committee for the Purpose of Control and Supervision (CPCSEA) were strictly followed throughout the execution of studies. Male Sprague–Dawley rats, weighing 250– 280g were used for conduction of in vivo experimentation, which were properly treated with customary existing conditions such as maintenance of 12h light and 12h dark timings, controlled temperature and humidity (19±29 °C; 35–60% humidity), standard pellet diet, water and beddings in polypropylene caging.
Study protocol consisted of division of 24 animals in four groups each group having 6 animals. Animals were given under mentioned treatment for induction of diabetes:-
A. Group 1: Animals were kept untreated (normal/nondiabetic) and given vehicles only
B. Group 2, 3 & 4: Animals were treated with single dose of streptozotocin (STZ, chemical name; 2-Deoxy-2-(3- methyl-3-nitrosoureido)-D-glucopyranose) i.e. 60mg/kg body weight in citrate buffer, pH 4.5, IP [16,17].
Animals were checked for glucose level with glucometer (OneTouch® Horizon™ Blood Glucose Monitoring System, Johnson & Johnson) equipped with enzyme (glucose oxidaseperoxidase) loaded strips, for this 1.5μL of blood was taken out with microlitre syringe from tail vain and putted on the strip. Blood glucose levels were consistently monitored upto 3 weeks with a frequency of 0, 3, 7, 14 and 21 days. According to guidelines, animals detected with blood glucose levels more than 300mg/ dL upto 3 weeks after the exposure were considered as diabetic, which was further evidenced by the glucometer readings which furnished values above 300mg/dL. Animals which had not shown glucose level above 300mg/dL were casted off. To evaluate the hypoglycemic activity of the prepared formulations, animals were given following treatment schedule. Group 1, as usual served as control with no such administration of RPG in any form, group 2 was administered with pure RPG 2mg/kg (distilled water dispersion), group 3 was administered with conventional/ marketed RPG formulation (2mg, Eurepa) and group 4 was treated with diluted RPG loaded SMEDD’s (equivalent to 2mg/ kg) by oral administration. Further blood samples of all animals were collected by using the method mentioned above, timings were kept at first 0min (pre dosing) followed by gradual gaping upto 24hrs [18]. Glucose levels in collected blood samples were measured using glucometer. Statistical analysis All experimental data was furnished as mean±standard error of the mean (S.E.M), and statistical analysis was executed by using One way analysis of variance (ANOVA).
Results and Discussion
Solubility studies
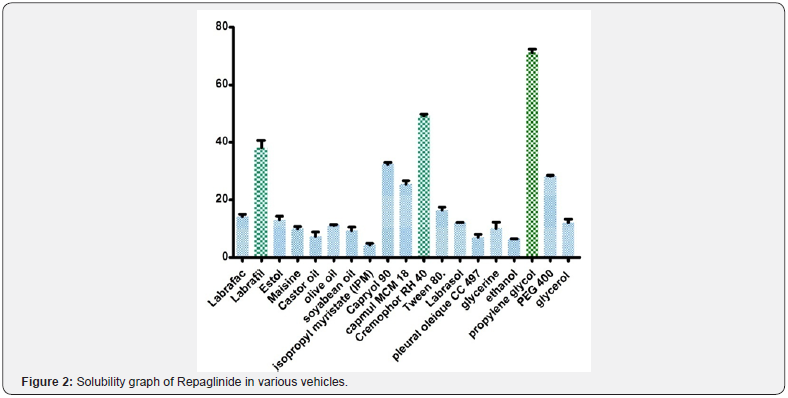
RPG concentration in a variety of excipients at 25 C was verified by HPLC. Chremophor RH40 and Propylene glycol provided higher solubility, which were used as surfactant and cosurfactant, and Labrafil was selected as the oil phase. The selection of high soluble components is very important for formulation of the optimal novel emulsion, with existence as a single phase. The solubility of RPG in various vehicles is presented in Table 3 and Figure 2 (mean±SD; n=3)
Preparation of pseudo-ternary phase diagrams
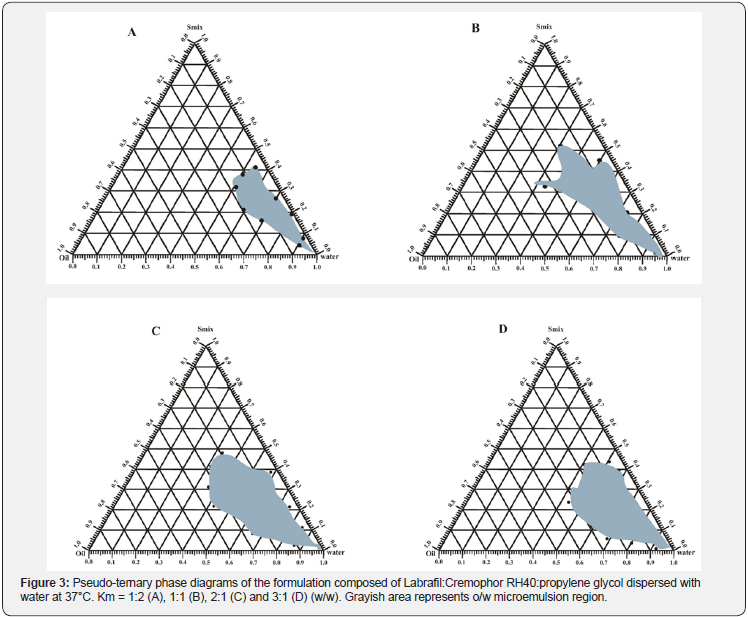
Area of microemulsion regions at 37 °C were detected by construction pseudo-ternary phase diagrams using water titration method. Formulations were added with purified water which was utilized as diluting medium. Investigation of appropriate ratio of one excipient to another in the SMEDDS formulation was done. Numerous formulations having different lipid and Km values (ratio of surfactant to co surfactant) were dispersed with water at 37 °C. Phase diagrams of the formulation consisting Labrafil, Cremophor RH40 and Propylene glycol, scaling with different Km values, are presented in Figure 3. Greyish part corresponds to o/w microemulsion existence region. Larger greyish part signifies better self-micro emulsification efficiency which was represented by the formulation having Labrafil as lipid in contrast to other formulations. Verification of most favorable ratio of the excipients in the formulation was also done with the help of phase diagrams. It was found out that when Km value was 1, the microemulsion region had the largest size.
Characterization of SMEDDS
Phase separation and visibility grade: The prepared SMEDDS formulations upon dilution with distilled water in ratio of 1:100 were investigated for phase separation and graded from A to E according to visibility grading system [19]. Formulation ME1, ME2 & ME3 showed no phase separation, precipitation and rapidly formed with a visibility grade A as shown in Table 4, hence were evaluated further.
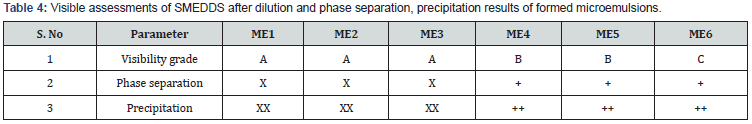
x: no phase separation; xx: no precipitation; +: phase separation; ++: precipitation.
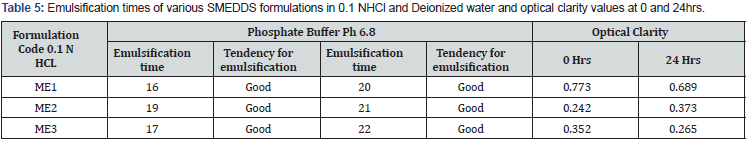
Determination of emulsification time and optical clarity: In dilution to aqueous medium SMEDDS formulation must disperse rapidly under gentle agitation, evaluation of determined formulation was done in both the media of 0.1 N HCL and pH 6.8 phosphate buffer shown in Table 5 which furnished emulsification time below 60 seconds with fair propensity for emulsification. It was also noticed that with the increment in oil proportion, the emulsification time increased due to larger volume of oil and less surfactant concentration increase in the interfacial tension decreases the rate of emulsification. Optical clarity values demonstrate no much considerable alterations in absorbance values at 0 and 24hrs of dilution Table 5, which clearly indicate stability of formulation at end of 24hrs.
Droplet size and zeta potential: SMEDDS formulations subjected to determination of effect of dispersing media on zeta potential, for this formulations were dispersed with deionized water and 0.1N HCl, correspondingly. Minor difference in zeta potential was observed between the two dispersing media at the same dilution. Detailed assessments of optimized formulations are summarized in Table 6
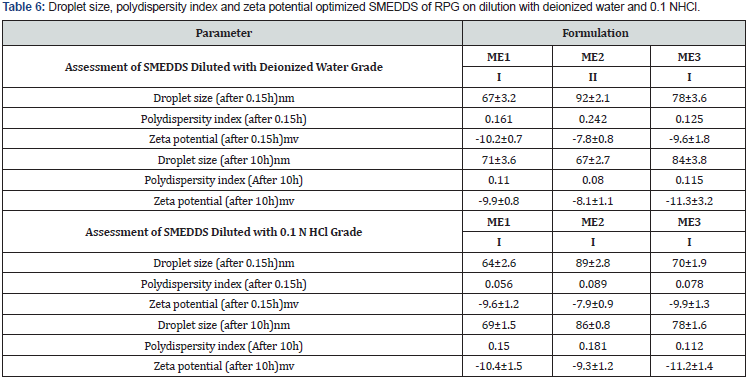
Cloud point measurement: Above cloud point temperature formulation clarity turns to cloudiness which is attributed to phase separation and drug precipitation of the emulsion. Cloud point should be above 37 °C since both the drug solubilization and stability of emulsion decreases with phase separation. The cloud point temperatures of different formulations determined were in the range of 64–78 °C. The reason for higher cloud point temperature may be attributed to solubility of drug in oil and surfactant system, optimized ratio of S/CoS and/or surfactants with higher HLB values; this infers good thermal stability of all the tested formulations.
Thermodynamic stability studies: Thermodynamic stability study assists in identification and avoidance of metastable SMEDDS formulations, in which formulation were subjected to different stress tests like centrifugation and freeze– thaw test. Further and frequent analysis was needed not to be executed during storage if prepared SMEDDS formulations could withstand this study. Content of surfactant (Chremophor RH40) in the formulation plays a crucial role in the thermodynamic stability of the formulations. All formulations were found to be stable in this study and were considered for further characterization.
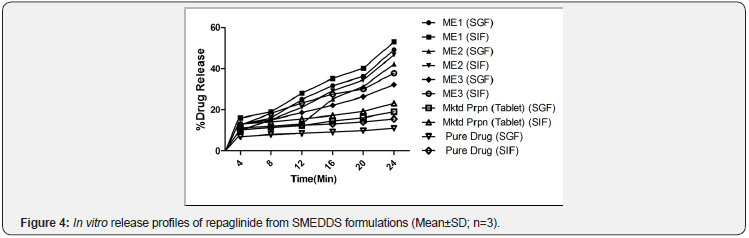
Drug release studies: After oral administration when SMEDDS come across aqueous medium, drug may present in free molecular state or in emulsion form or in solubilized micellar solution. In order to release from emulsion drug should undergo interfacial transport across surfactant layer coated around droplet, which further enters into surrounding aqueous medium by diffusion and convective transport [20]. It indicates when those fine oil droplets are dispersed in the medium and it will not lead to drug diffusion into aqueous medium from oil droplet instantaneously. Under these circumstances, it is necessary to separate free drug molecules from those entrapped in the emulsion droplets or micelles to assess the real drug release pattern [21]. However to facilitate the real drug release pattern the dialysis bag method with a molecular weight cut-off of 12,000 was utilized in drug release studies. The formulations were diluted with SGF & SIF to circumvent the sticking of formulation with the membrane as reported [22]. The drug release pattern of SMEDDS shown in Figure 4 reveals that in contrast to other 2 formulations along with marketed preparation and pure drug, the highest drug release was observed with ME1 formulation after 24h that could be due to proper compromise between proportions of oil and surfactant in the system. Though the formulation ME2 produced emulsions with better spontaneity and more clarity it showed 40% drug release this may be due to high Co surfactant proportion in the formulation. The formulation ME3 showed less drug release of around 50%, this may be due to higher proportion of oil resulting in larger droplet size, leading to lesser surface area exposed to the medium. The drug release pattern from ME1 formulation followed zero order. Based on the aforementioned results of spontaneity of emulsification, globule size analysis, stability and in-vitro drug release studies the formulation ME1 is considered to be the optimized formulation among the formulations studied.
Transmission electron microscopy: The morphology of microemulsion was examined with a transmission electron microscope. The droplet on the microemulsion appears dark with the bright surroundings. TEM photographs (Figure 5a-c) further conformed that the globules are spherical in shape.

Stability studies: Samples of RPG SMEDDS were charged on accelerated and long term stability conditions. Chemical and visual observations of samples were shown in Table 7. No significant change in the drug content in the formulations was observed over the period of 3 months at accelerated and longterm stability conditions.

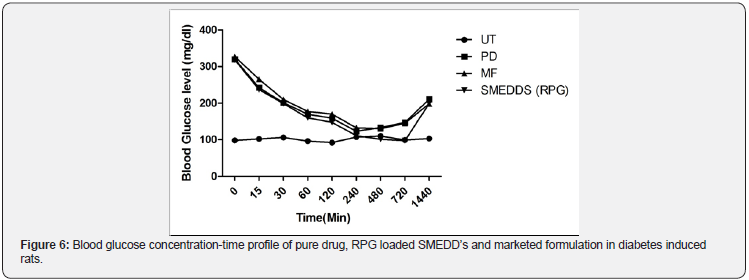
In vivo Study: Execution of determination of hypoglycemic activity was done by utilizing STZ-induced experimental diabetes in male Sprague dawley rats in a type 2 diabetes mellitus model. RPG acts by stimulating endogenous insulin secretion during meal by mimicking physiological insulin secretion pattern [23]. A comparative evaluation for hypoglycemic potential between pure RPG, conventional RPG tablets equivalent to 2mg and RPG loaded SMEDD’s was performed by blood glucose level measurement through glucometer strips in diabetic rats. Figure 6 shows blood glucose level readings monitored at different time intervals. Results suggested that at the end of 1hr blood glucose levels in untreated group was 96±1.2mg/dl whereas in Pure RPG drug, marketed formulation and SMEDD’s was 169.2±2.2mg/dl, 177.2±2.4mg/dl and 159.8±0.6 mg/dl respectively. At the end of 8th hour, Pure RPG drug (133.1±3.2mg/dl) and marketed formulation (130.1±0.4mg/dl) showed no decrement in blood glucose levels which indicates their completion of action, whereas RPG loaded SMEDD’s showed still reducing value (101.2±1.2mg/dl) which indicates not only its significant potential in hypoglycemic activity but also prolonged duration of action which may be imputed to elevated rate of absorption and increased plasma drug concentration in contrast to pure RPG drug and marketed formulation (Figure 6). From results, it is concluded that RPG loaded SMEDD’s had shown surpassing performance in contrast to pure RPG and marketed formulation, Hence administrating RPG in the form of SMEDD’s can significantly renders superior hypoglycemic performance and eventually bioavailability Figure 7.
Conclusion
An optimized RPG loaded SMEDDS formulation was successfully prepared consisting 24.04% v/v of Labrafil as lipid phase, 39.84% v/v of Chremophor RH40 as surfactant and 34.56% v/v of Propylene glycol as Co Surfactant. Above formulation was optimized on the basis of optimum globule size, zeta potential and Polydispersity index, emulsification and optical clarity, cloud point measurement etc. Results from stability studies at 25 °C±2 °C/60±5% RH & 40 °C±2 °C/75±5% RH indicated stability of RPG SMEDDS and there was no considerable changes in the observed physical parameters. Selected SMEDDS formulation ME1 showed highest % of invitro drug release in simulated gastric and intestinal fluid media in contrast to pure RPG drug and marketed RPG formulation. Results of in-vivo showed that developed SMEDDS formulation significant reduction in blood glucose levels in contrast to marketed formulation. Hence it is concluded that SMEDDS can be explored as a potential drug carrier for bioavailability enhancement of RPG and other lipophilic drug(s).
Acknowledgement
The authors would like to thank Gattefosse Pvt. Ltd (Mumbai, India), Croda Pvt. Ltd (Mumbai, India), Abitec Corp. & BASF (Mumbai, India) for providing the excipients for this study.
References
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5): 1047-1053.
- Whirl-Carrillo M, Mc Donagh EM, Hebert JM, Gong L, Sangkuhl K, et al. (2012) Pharmacogenomics Knowledge for Personalized Medicine. Clin Pharmacol Ther 92(4): 414-417.
- Zhua Z, Yangb T, Zhaoa Y, Gaoa N, Lenga D, et al. (2014) A simple method to improve the dissolution of repaglinide and exploration of its mechanism. Asian J Pharm Sci 9(4): 218-225.
- Charman WN (2000) Lipids, lipophilic drugs, and oral drug deliverysome emerging concepts. J Pharm Sci 89(8): 967-978.
- Wasan KM (2001) Formulation and physiological and biopharmaceutical issues in the development of oral lipid-based drug delivery systems. Drug Dev Ind Pharm 27(4): 267-276.
- Shah NH, Carvajal MT, Patel CI, Infeld MH, Malick AW (1994) Self emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm 106(1): 15-23.
- Farah N, Laforet JP, Denis J (1994) Self-micro emulsifying drug delivery systems (SMEDDS) for improving dissolution and bioavailability of poorly soluble active ingredients. BT Gattefossé 87: 41-47.
- Craig DQM, Barket SA, Banning D, Booth SW (1995) An investigation into the mechanisms of self-emulsification using particle size analysis and low frequencies dielectric spectroscopy. Int J Pharm 114(1): 103- 110.
- Pouton CW (1985) Self-emulsifying drug delivery systems: assessment of the efficiency of emulsification. Int J Pharm 27(2): 335-348.
- Khoo SM, Humberstone AJ, Porter CJH, Edwards GA, Charman WN (1998) Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine. Int J Pharm 167(1-2): 155-164.
- Gershanik T, Benita S (2000) Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm 50(1): 179-188.
- Kang BK, Lee JS, Chon SK, Jeong SY, Yuk SH (2004) Development of self-micro emulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm 274(1-2): 65-73.
- Grove M, Müllertz A, Nielsen JL, Pedersen GP (2006) Bioavailability of seocalcitol II: Development and characterisation of self-micro emulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides. Eur J Pharm Sci 28(3): 233-242.
- Bandivadeka MM, Pancholi SS, Kaul-Ghanekar R, Choudhari A, Koppikar S (2012) Self-microemulsifying smaller molecular volume oil (Capmul MCM) using non-ionic surfactants: a delivery system for poorly water-soluble drug. Drug Dev Ind Pharm 38(7): 883-892.
- Dixit AR, Rajput SJ, Patel SG (2010) Preparation and Bioavailability Assessment of SMEDDS Containing Valsartan. AAPS Pharm Sci Tech 11(1): 314-321.
- Ghosh S, Bhattacharya S, Rashid K, Sil PC (2014) Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol Rep 2: 365-376.
- Ganda OP, Rossini AA, Like AA (1976) Studies on streptozotocin diabetes. Diabetes 25(7): 595-603.
- Bera K, Khanam J, Mohanraj KP, Mazumder B (2014) Design and evaluation of mucoadhesive beads of glipizide as a controlled release drug delivery system. J Microencapsul 31(3): 220-229.
- Qi X, Wang L, Zhu J, Hu Z, Zhang J (2011) Self double emulsifying drug delivery system (SDEDDS): A new way for oral delivery of drugs with high solubility and low permeability. Int J Pharm 409(1-2): 245-251.
- Zhang P, Liu Y, Feng N, Xu J (2008) Preparation and evaluation of self micro emulsifying drug delivery system of oridonin. Int J P 355(1-2): 269-276.
- Wu W, Wang Y, Que L (2006) Enhanced bioavailability of silymarin by self micro emulsifying drug delivery system. Eur J Pharm Biopharm 63(3): 288-294.
- Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, et al. (2007) Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm 66(2): 227-243.
- Thomsen MK (1999) Mechanisms of action of repaglinide at a cellular level. Diabetes Metab 7: 3-11.






























