Innovative Technologies for Gastro-Retentive Drug Delivery Systems
Vijay Chudiwal1*, Sadhana Shahi2, Swapnil Chudiwal2 and Dheeraj Ahale2
1Wockhardt Research Centre, India
2Government College of Pharmacy, India
Submission: September 22, 2017; Published: February 23, 2018
*Corresponding author: Vijay S Chudiwal, Wockhardt Research Centre, India, Tel: +91-9175746701; Email: vijaychudiwal@gnail.com
How to cite this article: Vijay Chudiwal, Sadhana Shahi, Swapnil Chudiwal, Dheeraj Ahale. Innovative Technologies for Gastro-Retentive Drug Delivery Systems. Glob J Pharmaceu Sci. 2018; 4(4): 555650. DOI: 10.19080/GJPPS.2018.04.555650
Abstract
Gastro-retentive drug delivery is most likely utilized by many pharmaceutical industries in view of its commercial success. Drugs that are primarily absorbed in the stomach, short half life and poorly soluble at alkaline pH are the most suitable candidate for the development of gastro- retentive drug delivery system. Traditional approaches have been followed to encourage gastric retention of an oral dosage form. However, over the past decades the pursuit and exploration of devices designed to be retained in the upper part of the gastrointestinal (GI) tract has advanced consistently in terms of technology and diversity. A number of major drug companies have focused efforts on the design of gastric retention technologies such as Oleotec, Soctec, Accordion Pill, GRID, Multiple Polymers Hydrophilic Matrix, Acuform, GIPET, GIRES, Micropump and Gastrodose. This review article highlights the recent commercially available gastro-retentive technologies in the field of GRDDS.
Keywords: Gastro-Retentive; Oleotec; Soctec; Acuform; Micropump
Introduction
Oral drug delivery is the ideal and well preferable route of administration due to its simple and comfortable use and flexibility about different types of formulation. Safest and convenient way of medication administration is achieved by oral route with highest patient compliance. More than 60% of commercial drugs available in the market use oral administration for the delivery [1]. During the last five decades life span, various oral delivery systems have been developed to act as a drug reservoir from which the active substance is released over a prolonged period of time and at controlled release rate [2]. However, there are several evidences shows that in vivo drug release of solid oral controlled released dosage form is unpredictable despite its excellent in vitro release profile [3].
Gastro-retentive dosage forms are delivery systems that will provide the system to be able to control the gastric residence time or gastric transit time of the dosage form to achieve a prolonged and predictable drug delivery profile in the upper part of the Gl-tract [4]. Traditional approaches have been followed to encourage gastric retention of an oral dosage form. Briefly, gastro-retentive systems can be based on the buoyant (floating) systems, bioadhesive or mucoadhesive systems and systems that have a size or will expand in the stomach to a size that is too large to pass the pyloric sphincter [5]. Over the past three decades, the pursuit and exploration of devices designed to be retained in the upper part of the gastrointestinal (GI) tract has advanced consistently in terms of technology and diversity [6]. A number of major drug companies have focused efforts on the design of gastric retention technologies as outlined below.
Oleotec™ and soctec™
Oleotec™ and Soctec™ gastro-retentive technology are developed by Skyepharma. Oleotec™ is specially designed for drugs requiring high therapeutic doses not compatible with conventional dosage forms. This system offers for drugs that are primarily absorbed in the upper segments of the GI tract or with a localized effect. Oleotec™ is basically a gel supplied in stick pack which forms continuous structure at surface of stomach content. The hydrophobic nature of system provides sustained release effect and limits disaggregation of system in stomach. Soctec™, was developed for drugs that need to be retained in the stomach for an extended period of time to then be slowly released either for a local effect in the stomach or for absorption in the upper intestine so that it can be delivered in a controlled way to the duodenum and jejunum for subsequent absorption, so improving oral bioavailability and duration of action for sustained release formulations. Soctec™ is an easy to swallow elongated capsule. It has an integral buoyancy chamber and a counter-balanced denser ballast to ensure that it floats upright in the stomach fluids as soon as it is swallowed (Figure 1).
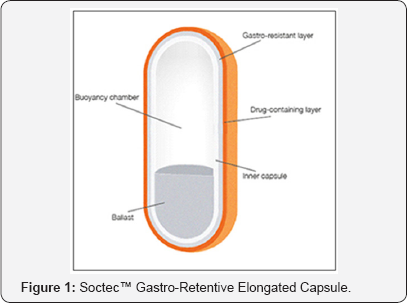
Normally the contents of the stomach are crushed by peristaltic contractions and the contents are rapidly expelled through the pylorus into the duodenum. However, owing to its shape and physical properties Soctec™ could be retained in the stomach for more than nine hours. During this time Soctec™ releases its drug load at a predetermined rate, allowing absorption in the small intestine's upper segments. Soctec™ can be used with a range of drugs that have a narrow absorption window and are preferably absorbed in the upper intestine segments. It can also improve the bioavailability of drugs that are degraded by the alkaline pH of the lower gastrointestinal tract. Soctec™ is very adaptable and can be used with drugs having different physicochemical and therapeutic characteristics. Soctec™ utilises conventional manufacturing techniques facilitating rapid and cost-effective industrialization [7].
Accordion Pill™ technology
The Accordion Pill™ is a unique gastro retentive formulation composed of pharmaceutical biodegradable polymeric films. It is a multi-layer, planar structure, folded to an accordion shape into a standard size, regular capsule. Upon reaching the stomach, the capsule dissolves; the Accordion Pill™ unfolds and is retained in the stomach for up to 12 hours, under regular calorie diets. While in the stomach, the Accordion Pill™ releases the drug in a controlled manner towards the upper part of the gastrointestinal tract which enables prolonged and continuous absorption phase of the drug in the upper part of the gastrointestinal tract, resulting in improved efficacy and safety profile, as well as reducing frequent daily dosing (Figure 2).

The drug release mechanism is independent of the Accordion pill™ retention mechanism Once the Accordion Pill™ is expelled from the stomach; it is fully degraded in the intestine. The Accordion Pill™ can combine immediate and controlled- release profiles and can contain more than one API and feasible for high drug loading, up to 550mg. The Accordion Pill™, a novel gastro-retentive delivery system, significantly improves the Pharmacokinetics of drugs with either narrow absorption window or poorly soluble drugs that belong to Biopharmaceutics Classification System (BCS) class II and Class IV. The Accordion Pill™ is designed for drugs that are characterized by Narrow Absorption Window (poor colonic absorption), Poor solubility, BCS class II or IV, Act locally, in the stomach or in the upper part of the GI tract and adverse effects correlate with the drug reaching the distal parts of the GI tract. Carbidopa/Levodopa and Zaleplon are under development. The Accordion Pill™- Zaleplon (AP-ZP) is designated to provide a fast onset of sleep, good sleep maintenance and to avoid "next day" residual side effects. Gastro-retention of Zaleplon provides significantly prolonged absorption phase of the drug and reduced variability, by absorption through a defined, specific site, in the upper part of the GI tract [8].
Gastro Retentive Innovative Device (GRID)

Gastro Retentive Innovative Device (GRID) is an ideal once- a-day system for drugs that are otherwise absorbed only in stomach or small intestine. GRID is designed so that drug is retained in the stomach for over an eight-hour span. Longer retention in stomach improves the drug absorption. The tablet offers a combination of instant and sustained drug release profiles, and being once a day improves patient compliance. Based on GRID (Figure 3), Baclofen GRS, a once-a-day capsule to treat muscle spasticity is marketed in India. Some drugs cannot be formulated as controlled release dosage forms because of poor solubility, degradation in the alkaline media, or presence of specific transport mechanism in the small intestine. This leads to the presence of a 'window' or 'narrow zone’ of absorption. GRID is designed to offer longer retention times in the stomach, of about eight hours, for such drugs. This innovative system is a dosage form with specialized multiple coatings. On ingestion of the dosage form along with food, it floats instantaneously on the gastric contents. GRID's coatings are activated by gastrointestinal fluid, eventually leading to swelling, to about eight to eleven times its initial volume. During the cycle of intense gastric movements, GRID retains its shape and form so as to release medication in a controlled fashion. Plasma concentrations for medicines are thus maintained in the therapeutic range for a prolonged period; hence this dosage form can be used as a "Once-a- day" system. Specific release profiles for drugs can be tailored to achieve combination of immediate and slow release using this innovative dosage form. Retention of the dosage form close to its site of absorption may help in reducing the dose and thus the side effects [9].
Multiple polymers hydrophilic matrix technology
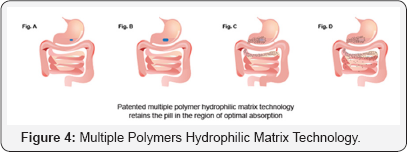
Cetapin XR the Sanofi formulation of Metformin XR uses a patented multiple polymer hydrophilic matrix technology as a gastroretentive delivery system, to achieve prolonged release of Metformin hydrochloride. The polymers are a novel combination of non ionic and ionic hydrophilic polymers. Metformin hydrochloride granules are compressed into tablets along with the polymers in a ratio that is optimized to provide pH independent drug release from the tablet. The unique releasecontrolling properties of the polymer compounds allows for a gradual and complete release of Metformin hydrochloride from stomach to jejunum, unaffected by gastric pH fluctuations (Figure 4). When the tablet comes in contact with the gastrointestinal fluids (Figure 4a), Metformin imbibes water hydrates and forms a gelatinous swellable matrix (Figure 4b). The drug release from the matrix occurs via a process of dissolution of the drug and subsequent diffusion through the gel matrix in a controlled manner (Figure 4c). The matrix controls the rate and extent of release of Metformin XR (Figure 4d). As the tablet swells, it is retained in the stomach and upper intestines for a longer time, thereby providing maximum drug available at the site of absorption (Figure 4d). This technology has given consistent and reproducible results providing: Optimal absorption; Less irritation; Improved plasma levels; and better bioavailability [10].
Acuform® technology
Acuform® is Depomed's patented, polymer-based technology designed to optimize drug delivery. This technology allows for targeted, controlled delivery of pharmaceutical ingredients to the upper gastrointestinal (GI) tract, the preferential absorption site for many oral drugs. Unlike immediate release and some extended release formulations that pass through the upper GI tract within approximately three hours following ingestion, Acuform technology's unique swelling polymers allow the tablet to be retained in the stomach for approximately eight to ten hours. During this time, the tablet's active ingredient is steadily delivered to the upper GI tract at the desired rate and time. This gradual, extended release allows for more of the drug to be absorbed in the upper GI tract, offering the potential for greater treatment efficacy and increased treatment tolerability with the convenience of once- or twice-daily dosing. This technology is used in the formulation of Depomed's Nucynta®ER (Tapentadol ER Tablets) and once-daily Gralise (Gabapentin) tablets [11], as well as in two products marketed by Salix’s Glumetza® (Metformin HCl ER Tablets) and Merck's Janumet® XR (Sitagliptin and Metformin HCl ER).
Tablets utilizing this technology can be tailored to deliver new drug combinations of varying properties, either simultaneously or sequentially, for a wide array of product possibilities. In particular, this technology may prove to be an effective delivery solution for compounds that are absorbed in the upper GI region, Insoluble in water, Available through active transport mechanisms, Irritating to the mucosa of the small intestines, imbalancing, irritating, or unsafe in the lower GI region and More effective when plasma levels have less fluctuation. This technology incorporates standard, inexpensive pharmaceutical excipients that are on the FDA’s inactive ingredients list. The manufacturing process utilizes standard high-speed tableting equipment. Importantly, the improvements made possible with Acuform delivery technology, including reduced dosing requirements, improved efficacy and decreased toxicity; hold the potential to provide patented NCE-like differentiation to already approved therapeutics. These factors make the Acuform technology a potentially attractive option for companies interested in developing improved formulations of off-patent drugs [12].
Gastrointestinal permeation enhancement technology
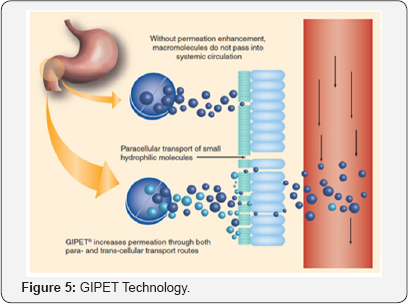
Merrion Pharmaceutical’s Gastrointestinal Permeation Enhancement Technology Enhancement Technology (GIPET) is a unique approach allows drugs that currently can only be given parenterally (injectable) to be converted into oral (tablet/capsule) forms, as well as improving the absorption of current oral drugs (Figure 5). GIPET uses specifically designed oral formulations of patented absorption enhancers which activate micelle formation facilitating transport of drug and substantially increasing absorption with good reproducibility and a strong safety profile [13].
Gastrointestinal Retention System (GIRES)
GIRES™ is a gastro-retentive technology that gives 16-24 hour retention times in the stomach, without food having a detrimental effect. GIRES comprises a controlled-re lease dosage form inside an inflatable pouch, which is placed in a drug capsule for oral administration. Upon dissolution of the capsule, a gasgenerating system inflates the pouch in the stomach where it is retained for 16-24 hours, all the time releasing agents described herein. Merrion's developed another GIRES technology that consists of a controlled-release dosage form inside an inflatable pouch, which is placed in a drug capsule for oral administration. Upon dissolution of the capsule, a gas generating system inflates the pouch in the stomach. In clinical trials the pouch has been shown to be retained in the stomach for 16-24 hours [14].
Micropump technology
Flamel's Micropump® platform permits either extended, or both delayed and extended, delivery of small molecule drugs via the oral route. Micropump consists of a multiple-particulate system containing 5,000 to 10,000 microparticles per capsule or tablet. The 200-500 microns diameter-sized microparticles release the drug at an adjustable rate and over an extended period of time. Micropump’s key attributes includes extended release in the GI tract allowing mean plasma residence times to be extended for up to 24 hours, potentially improved efficacy (by extending therapeutic coverage), potentially reduced toxicity and/or side effects (by reducing Cmax or peak drug concentration in the plasma, or by reducing intra- and interpatient variability), improved patient compliance (by reducing frequency of administration), applicable to poorly soluble (<0.01mg/L) as well as highly soluble (>500g/L) and to low dose (e.g. 4mg) or high dose (e.g. 1,000mg) drugs, excellent mouth feel, taste masking properties [15].
GastroDose technology
Gastrodose is retained in the stomach for extended periods of time used for the treatment of disorders of the stomach or upper gastrointestinal tract. It is also suited for drugs that are readily absorbed into the circulation from the stomach or upper small intestine. For instance, Alza Corporation has developed a gastro-retentive platform for the OROS™ system, which showed prolonged gastric residence time in a dog model as the product remained in the canine stomach at 12 hours post dose and was frequently present at 24 hours. In humans, in the fasted state, the average gastric residence time for the same system was 33 minutes. DepoMed has developed technology that consists of a swellable tablet. After ingestion of the tablet, it swells and achieves sufficient size to resist gastric emptying, while simultaneously providing controlled release of the drug. Two of the products that DepoMed is developing include Metformin GR™ and Ciprofloxacin GR™ [16].
Conclusion
Innovative technologies for gastro-retentive drug delivery systems present indubitable benefits for drug administration. This review attempts to compile latest innovative technology regarding gastroretentive drug delivery systems developed by pharma companies. As seen, the effort to produce these new gastro-retentive technologies systems by pharmaceutical industries is clearly high. Based on the investigations on current innovative technologies, it can be concluded that gastro- retentive system could be marked as the best suited for potential drug candidate. Recent technological breakthroughs in drug development have resulted in innovative therapeutics leading to novel products with higher advantages and lower side effects. In conclusion, pharmaceutical industries are now more interested in the development of innovative gastro-retentive drug delivery technologies to strengthen their product pipeline.Innovative technologies for gastro-retentive drug delivery systems present indubitable benefits for drug administration. This review attempts to compile latest innovative technology regarding gastroretentive drug delivery systems developed by pharma companies. As seen, the effort to produce these new gastro-retentive technologies systems by pharmaceutical industries is clearly high. Based on the investigations on current innovative technologies, it can be concluded that gastro- retentive system could be marked as the best suited for potential drug candidate. Recent technological breakthroughs in drug development have resulted in innovative therapeutics leading to novel products with higher advantages and lower side effects. In conclusion, pharmaceutical industries are now more interested in the development of innovative gastro-retentive drug delivery technologies to strengthen their product pipeline.Innovative technologies for gastro-retentive drug delivery systems present indubitable benefits for drug administration. This review attempts to compile latest innovative technology regarding gastroretentive drug delivery systems developed by pharma companies. As seen, the effort to produce these new gastro-retentive technologies systems by pharmaceutical industries is clearly high. Based on the investigations on current innovative technologies, it can be concluded that gastro- retentive system could be marked as the best suited for potential drug candidate. Recent technological breakthroughs in drug development have resulted in innovative therapeutics leading to novel products with higher advantages and lower side effects. In conclusion, pharmaceutical industries are now more interested in the development of innovative gastro-retentive drug delivery technologies to strengthen their product pipeline.Innovative technologies for gastro-retentive drug delivery systems present indubitable benefits for drug administration. This review attempts to compile latest innovative technology regarding gastroretentive drug delivery systems developed by pharma companies. As seen, the effort to produce these new gastro-retentive technologies systems by pharmaceutical industries is clearly high. Based on the investigations on current innovative technologies, it can be concluded that gastro- retentive system could be marked as the best suited for potential drug candidate. Recent technological breakthroughs in drug development have resulted in innovative therapeutics leading to novel products with higher advantages and lower side effects. In conclusion, pharmaceutical industries are now more interested in the development of innovative gastro-retentive drug delivery technologies to strengthen their product pipeline.
Acknowledgment
The authors were thankful to Wockhardt Research Centre, Aurangabad and Dr. Babasaheb Ambedkar Marathwada University, Aurangabad for providing the necessary support to carry out this survey.
References
- Sastry SV, Nyshadham JR, Fix JA (2000) Recent technological advances in oral drug delivery-a review. Pharmaceutical science & technology today 3(4): 138-145.
- Park K (2014) Controlled drug delivery systems: past forward and future back. Journal of Controlled Release 190: 3-8.
- Ratnaparkhi MP, Gupta Jyoti P (2013) Sustained release oral drug delivery system-an overview. Terminology 2(3): 11-14.
- Khan R (2013) Gastroretentive drug delivery system-a review. Int J Pharm Bio Sci 4(2): 630-646.
- Kumar MR, Satyanarayana B, Paladugu ND, Muddasar S, Pasha SI, et al. (2013) A comprehensive review on gastro retentive drug delivery system. Acta Chimica and Pharmaceutica Indica 3(2): 149-164.
- chudiwal V, Shahi S (2016) Patent perspective of advanced gastroretentive drug delivery systems. Research Journal of Pharmaceutical Dosage Forms and Technology 8(1): 15-23.
- Vectura (2017) Vectura Group plc: Oral drug delivery technologies.
- IntecPharma (2016) Technology-IntecPharma.
- Sunpharma (2016) Sun Pharma Advanced Research Company
- Sanofidiabetes (2013) Sanofi Cepatin XR | Sanofi Diabetes India.
- Yadav M, Sharma P, Chaudhary V, Srivastava B (2016) Gastroretentive Drug Delivery Systems: A promising approach. American Journal of Pharmaceutical Research 6(04): 2231-6876.
- Depomed (2016) Acuform® Drug Delivery Depomed.
- Walsh E, Adamczyk BE, Chalasani KB, Maher S, O'Toole EB (2011) Oral delivery of macromolecules: rationale underpinning Gastrointestinal Permeation Enhancement Technology GIPET1. Ther Deliv 2(12): 1595-1610.
- Evaluategroup (2013) Market intelligence leader in consensus 16. Wikinvest (2011) Drug Delivery Technologies for Penwest forecasts and analysis of the life science industry. Pharmaceuticals (PPCO).
- Rekhi GS (2010) Advances in solid dose oral drug delivery. ON Drug Delivery: Oral Drug Delivery and Advanced Excipients, Frederick Furness Publishing, The Candlemakers, UK, p. 1-36.
- Wikinvest (2011) Drug Delivery Technologies for Penwest Pharmaceuticals (PPCO).






























