Lipophilicity (LogD7.4) of N-Aryl Benzo Hydroxamic Acids
Ajita Dixit*
Pandit Ravishankar Shukla University, India
Submission: July 24, 2017; Published: February 21, 2018
*Corresponding author: Ajita Dixit, Pandit Ravishankar Shukla University, India, Email: ajita.dixit@gmail.com
How to cite this article: Ajita Dixit. Lipophilicity (LogD7.4) of N-Aryl Benzo Hydroxamic Acids. Glob J Pharmaceu Sci. 2018; 4(4): 555648. DOI: 10.19080/GJPPS.2018.04.555648
Abstract
Hydroxamic acids are polyfunctional molecules which show a wide spectrum of biological and medicinal activities. Lipophilicity is known to be important for absorption, permeability and in vivo distribution of organic compound. Lipophilicity is also a major structural factor that influences the pharmacokinetic and pharmacodynamic behaviour of compound.
Introduction
Lipophilicity is known to be important for absorption, permeability and in vivo distribution of organic compound [1]. Since about one century, it is recognized as a meaningful parameter in Structure-activity relationship studies and with the epoch making contributions of Hansch et al. [2] has become the single most informative and successful physico-chemical property in medicinal chemistry. Lipophilicity is defined "Lipophilicity represents the affinity of a molecule or a moiety for a lipophilic environment." Lipophilicity is determined experimentally as partition coefficients (written as logP and valid only for a single chemical species) or as distribution coefficients (written as logD referring to a mixture of chemical species generally pH dependent).
The importance of lipophilicity of molecule has been recognized in drug action including absorption, blood-brain distribution and drug-receptor interaction [3-4]. It has become a major experimental and theoretical tool in drug design. A detailed study of drug partitioning between polar (water/buffer) and an apolar (n-octanol) environment is very important since some drug physico-chemical properties and in vivo behaviour can be determined on the basis of the phenomenon [4]. During the last decades the n-octanol-water partition coefficients (logP or logD) was recognized as the standard hydrophobicity parameter to simulate drug partitioning in membranes and has been widely used in the field of QSAR and drug design [5].
Lipophilicity of a compound depends on various physical and chemical characteristics i.e., molecular surface area, molecular volume and polarity [6]. The major reason for the observed incompatibility of organic-solvent/bulk phase partitioning and membrane is that in a membrane a solute will not be disturbed homogeneously but rather, a gradient is formed that varies with the composition and geometry of membrane [7]. The high degree of ordering of solutes in a lipid bi layer compared with a bulk liquid phase also significantly changes the thermodynamic and partitioning. Never the less good correlation between the partition coefficient of various lipophilic compounds in membrane /buffer and octanol/water two phase's systems has been observed [8].
Materials and Methods
In the present investigation partition coefficient or distribution coefficient is determined experimentally by n-Octanol and buffer as solvent. ClogP is most widely used method for estimation of theoretical value of logP [9]. The ClogP method has improved considerably over the years to cover most neutral compound. Chemicals used are Specrtophotometric grade n-Octanol, Sodium Chloride, Disodium Hydrogen Phosphate Potassium Chloride and triple distilled water.
Measurement by Shake Flask Method
Distribustion coefficient (logD) of hydroxamic acid between n-Octanol and phosphate buffer (at 7.4) was determined at 25 °C by the shake flask method. Before partitioning of hydroxamic acid, the buffer (pH-7.4) and n-Octanol were saturated with each other .A carefully weighed (1mg) of hydroxamic acids was dissolved in octanol/buffer.
Results and Discussion
Distribution coefficient
Distribution coefficient LogD is calculated from following equation [4], LogD=Coct- Cbuffer/ Cbuffer x Vbuffer /Voct [1].
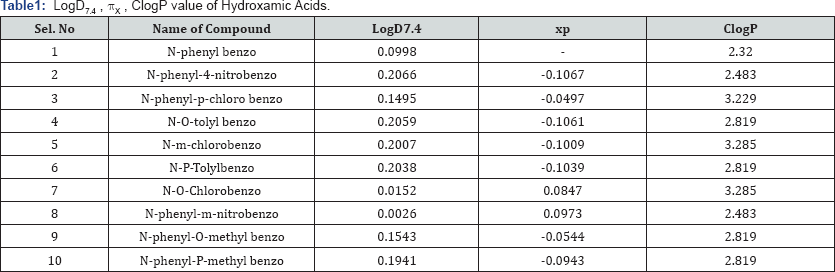
Where, Coct is concentration of octanol and Cbuffer is concerntration of buffer and Vbuffer is the volume of buffer (10ml) and VOCT is volume of Octanol (5ml). The data of all ten compounds are reported in Table 1.
Hydrophobic Parameter of Hydroxamic Acid
Hydroxamic parameter is calculated by expression,
πx = LogDx-Log DH [2]
πx = Hydrophobic parameter
Dx = distribution coefficient of parent compound
DH = distribution coefficient of derivatives.
The reference system is n-octanol /buffer. The value is given in calculation of ClogP data.
Calculation of ClogP Data
The ClogP program is based on the fragmental method developed by Leo & Hansch [4] and has become the standard in the field of rational drug design. 'Fragment Constant' method is based on chemical structure of the compound processed. It divides molecules into fragments and uses the constants of these fragments and correlation factors taken from its data for logP calculation method [9]. The Bio-loom program of BioByte Corp. was utilized to calculate ClogP for ten hydroxamic acids. The values are reported in Table 1.
Conclusion
A detailed study of drug partitioning between polar (water/ buffer) and an apolar (n-octanol) environment is very important since some drug physico-chemical properties and in vivo behaviour can be determined on the basis of the phenomenon. The values of lipophilicity, logD7.4, are in the range from0.0152.o 0.2066. The minimum value is obtained for N-Phenyl- 4-nitrobenzohydroxamic acid and maximum is for N-o-Tolyl Benzohydroxamic acid. The hydrophobic substitution constant nx are in the range from-0.1067 to 0.0973.
References
- Walter WP, Ajay, Murcko MA (1999) Recognizing molecules with druglike properties. Curr Opin Chem Bio 3(4): 384-387.
- Helmer F, Kiehs K, Hansch C, Biochem (2002) The linear free-energy relationship between partition coefficients and the binding and conformational perturbation of macromolecules by small organic compounds 7(8): 2858-2863.
- Avila CM, Martinez F, Bul CP (2003) Thermodynamics of Partitioning of Benzocaine in Some Organic Solvent/Buffer and Liposome Systems. 51(3): 237-240.
- Hansch C (1981) The physicochemical approach to drug design and discovery (QSAR) Drug Dev Res 1: 267-309.
- Testa B, Crivori P, Reist M, Carrupt PA (2000) The influence of lipophilicity on the pharmacokinetic behavior of drugs: Concepts and examples. Per Drug Discov Des 19(1): 179-211.
- Leo A, Hanch C, Jow PYC (1976) Dependence of hydrophobicity a polar molecules in the molecular volume. J Med Chem 19(5): 611-615.
- Pavelic Z, Skalko-Basnet N, Jalsenjak I (2004) Liposomal gel with chloramphenicol: characterisation and in vitro release. Acta Pharm 54(4): 319-330.
- Giagins C, Theocharis S, Kakoulidou AT (2007) Octanol/water partitioning simulation by reversed-phase high performance liquid chromatography for structurally diverse acidic drugs: Effect of n-octanol as mobile phase additive. J Chrom A 1166(1-2): 116-25.
- Takas-Novek K (1998) Computerized logP prediction using fragment methods. Acta, Pharm Hung 68(1): 39-48.






























