Antifungal Activities of Copper Surfactants Derived from Neem (Azadirecta indica) and Karanj (Pongamiapinnata) Oils: A Pharmaceutical Application
Shema Khan1, Rashmi Sharma2 and Arun Kumar Sharma2*
1Department of Chemistry, Govt. P.G. College Dausa, India
2Department of Chemistry, S.P.C. Govt. College Ajmer, India
Submission: July 12, 2017; Published: August 04, 2017
*Corresponding author: Arun Kumar Sharma, Department of Chemistry, S.P.C. Govt. College Ajmer, India.
How to cite this article: Shema K, Rashmi S, Arun K S. Antifungal Activities of Copper Surfactants derived from Neem (Azadirecta indica) and Karanj (Pongamiapinnata) Oils: A Pharmaceutical Application. Glob J Pharmaceu Sci. 2017; 3(4): 555616.
Abstract
Different methods were applied on Neem and Karanj oils; their Copper (II) soaps and also their Copper (II) soaps benzothiazole complexes to study their comparative fungicidal study. To find the biological activity different methods were used i.e. Antifungal Disk Diffusion Susceptibility Testing of Yeasts; approved Guideline (M 44-A, NCCLS, USA) and antifungal testing of Alternaria alternata suggested by Booth and Hawks worth. The testing was done on two species of Candida i.e. C.albicans and C.krusei. Antimicrobial disks used were Fluconazole, Amphotericin, Clotrimazole and Ketoconazole. These antimicrobial disks were used as standard, as the strains of Candida albicans and Candida krusei are susceptible to them. The diameter of zones of testing disks was compared with the zone diameter of these standards antimicrobial agents.
Keywords: Copper Neem-2-amino-6-methyl benzothiazole complex; Copper Neem soap; Copper Pongamia soap; Copper Pongamia-2-amino-6-methyl benzothiazole complex; Methylene blue dye; Mueller hinton agar
Abbreviations: CN: Copper Neem; CP: Copper Pongamia soap; CNB: Copper Neem-2-amino-6-methyl benzothiazole complex; CPB: Copper Pongamia-2-amino-6-methyl Benzothiazole complex; GMB:Methylene Blue Dye; MHA: Mueller Hinton Agar
Introduction
Recently, the deeper understanding of the role of metal ion in bio-system had led to the awareness that metal complexing is useful in the treatment of bacteria, fungal and viral diseases. Most of the antimicrobial agents are obviously organic compounds, which behave as good-chelating agent [1-3]. Their pathogenic activity is enhanced on complexing with various transition, inner transition and toxic metals [4-5]. The biological effect of their derivatives depends on the nature and structure of ligands and their metal complexes and also on the presence of particular element [6]. Many Copper compounds are employed as pesticides either alone [as Cu (I) oxide and Cu (II) sulphate] or in mixture. Examples of the latter are the pesticide Cupronil which contains 35% Cu (II) Carbonate hydroxide, the fungicide Cuprozan containing 37.5% Cu (II) Chloride hydroxide and 15% Zineb (zinc ethylene-bis-dithiocarbamate), Bordeaux mixture a fungicide prepared by reacting Cu (II) sulphate with hydrated lime [7].
Nitrogenous ligands have been found to be effective against many metal enzymes, bacteria and number of fungi [8]. Large number of compounds containing nitrogen and sulphur atoms in the heterocyclic ring shows different types of activities. Some examples are given here possessing benzothiazole or similar structure containing nitrogen and sulphur atoms in the aromatic cyclic ring [9].
• 8-methoxy-2-oxo-2H-Pyrimido [2,1-b] benzothiazole- 3-carbonitrile: used as ulcer inhibitor.
• 3-H-1, 2, 4-thiazole [3,4-b] benzothiazole-3-one: used as a fungicide.
• Thiazole derivatives: used as Pharmaceutical and agrochemical intermediates.
• S-methyl benzo [1,2,3] thiadiazole-7-carbothioate derivative used against a range of fungal infections of crops.
Recently, Wood has developed metabolic antagonist theory of drug action and Fields led to the synthesis of several coordination compounds, which have been used for antibacterial and anti-tubercular activity [10]. Synthesized and evaluated biological activities of some new 2-arylamino-4-fluoroaryl thiazoles were found to possess fungicidal, herbicidal and anti-arthritic activities [11]. Thus on the basis of the foregoing studies, it becomes very clear that benzothiazole and their substituted derivatives have been found to be very active against variety of bacteria, fungi, herbs and insects [12].
Some medicated soaps have been prepared earlierwith the antifungal agent such as Clotrimazole, Bifonazole, Miconazole, Ketoconazole, Itraconazole, Econazole, Oxiconazole, Fluconazole, Sulconazole, Fenticonazole, Haloprogin, Nystatin, Tolnaftate, Griseofulvin, Amphotericin B etc., [13]. It has also been established by several workers that chelation of the benzothiazole and there substituted derivatives enhances the biological and fungicidal activity [14]. The use of standard methodology for determination of antifungal activity of natural products against medical yeasts candida sp and cryptococcus sp. has been reported by many scientists earlier [15].
The N-donor ligand used during the present investigation is 2-amino-6-methyl benzothiazole, which has been very well reported to possess biocidal activity. Our continuing interest in the search for good fungicides and bactericides has led us to synthesize new complexes derived from Copper (II) Neem and Pongamia soaps with the above mentioned ligands and screen them for their fungicidal activities. Antifungal Disk Diffusion Susceptibility Testing of Yeasts; approved Guideline (M 44-A, NCCLS, USA) [16,17].
Experimental
The Method described here is intended for testing Candida Species and does not currently encompass any other genera and has not been used in studies of the yeast form of dimorphic fungi, such as Blastomyces dermatiditis or Histoplasma capsulatum. Moreover testing of filamentous Fungi (i.e. moulds) is not addressed in current procedure.
Methodology for the Disk Diffusion Test
Reagents & requirements
Media: Mueller-Hinton Agar No.2 + 2%Glucose+0.5mg/ ml Methylene blue dye (GMB) Medium: The agar medium had pH between 7.2 & 7.4 at room temperature after gelling. The sterilized media was poured in sterilized Petri-plates and after gel was set it was dried in an incubator with the lids ajar until moisture has evaporated (20-30min). i.e. the surface of agar was kept moist but with no droplets on the agar surface or the Petridish cover and then kept for 24hrs in other incubator at 37 °C for sterility test.
Preparation of mueller hinton agar (MHA)
a. MHA was prepared from a commercially available dehydrated MHA base according to the manufacturer’s instruction.
b. 0.1gm of methylene blue dye was dissolved in 20ml of distilled water and warmed gently. 100ml of this solution per litre of agar suspension was added. Then 20gms of glucose per liter of agar suspension was added and Autoclaved.
c. Immediately after autoclaving, the agar solution was allowed to cool at 45 to 50 °C in a water bath. Thereafter the freshly prepared and cooled medium was poured into flat bottomed glass Petri-plates on a horizontal leveled surface to give a uniform depth of approximately 4mm.
d. The agar medium was cooled to room temperature.
e. Plates were examined for sterility by incubating at 37 °C for 24 hours.
Reference strains for quality control: To Control the precision (repeatability) and accuracy (trueness) of the results obtained with disk diffusion test procedure, several quality control strains were obtained from a reliable source and were
a. Candida albicans ATCC 90028
b. Candida krusei ATCC 6258
Strong Quality Control Strains:
I. The quality control strains were tested by the standard disk diffusion test procedure using the same materials and methods that are used to test clinical isolates.
II. Quality control strains were stored in a way that minimizes the possibility of mutation in the organism.
III. There are several methods for prolonged storage of reference strains. Yeasts were grown on slants of potato dextrose agar and then frozen at 70 °C.
IV. Working quality control cultures were stored on sabouraud dextrose agar at 2 to 8 °C and sub cultured each for three successive weeks.
V. A quality control strain can be used to monitor the precision (repeatability) and accuracy (trueness) of the disk test as long as there is no significant change in the mean zone diameter that cannot be attributed to a faulty methodology.
Dispensing disk
I. Antimicrobial disks which were used as standard for quality control.
a) Fluconazole
b) Clotrimazole
c) Amphotericin
d) Ketoconazole
II. Prepared disks of the testing compounds.
III. To prepare the desks of testing compounds we required
o Whattmann filter paper No. 2.
o Sterile vials.
Firstly 6mm disks were punched and 100 disks per sterile vial plugged with cotton was kept in oven for sterilizing it. Number of vials was as many as per testing solutions of different conc. of solutions made. 0.5ml of these solutions each is dispensed in sterile vials containing 100 disks, so as all the solution in absorbed by 100 disks.
Procedure
Inoculation Preparation: Direct Colony Suspension Method: Steps for Preparation of the inoculum are as follows
I. All organisms were sub-cultured onto sabouraud dextrose agar to ensure purity and viability. The incubation temperature throughout was between 38 °C.
II. Inoculum was prepared by picking five distinct colonies of approximately 1 mm in diameter from a 24 hour old culture of Candida Species. Colonies were suspended in 5ml of Sterile 0.145mol/litre saline (8.5g/l NaCl 0.85% saline)
III. The resulting suspension was vortexed for 15 seconds and its turbidity was adjusted visually and also with a spectrophotometer by adding sufficient sterile saline or more colonies to adjust the transmittance to that produced by a 0.5 McFarland standard # at 625nm wavelength. This procedure will yield a yeast stock suspension of 1 X 106 to 1 X 105 cells per ml and produced semi-confluent growth with most Candida species isolates.
Preparation of McFarland nephlometry standard (0.5): To standardize the inoculum density for a susceptibility test, a BaSO4 suspension with turbidity equivalent to 0.5 McFarland standards or its optical equivalent was used as turbidity standard for inoculum.
Preparation
I. Required Reagents
a) BaCl2.2H2O 0.048M/lit or 1.175% (w/v)
b) H2SO4 0.18M/Lit or % (v/V)
c) Distilled water (DW) 200ml
II. Procedure
a) 100ml DW is taken in each two 250ml capacity sterile flasks.
b) In one flask, 1.175% BaCl2.2H2O Solution is prepared.
c) From the other flask, 1ml of DW is discarded and 1ml pure H2SO4 is added to make a 1% (v/V) solution.<
d) From the H2SO4 solution, 0.5ml is discarded and to the remaining 99.5ml, 0.5ml BaCl2.2H2O solution is added drop by drop with a constant stirring
e) The solution thus prepared is now consisting of 0.5 standards of McFarland standards which is equivalent to 1.5 X 108 cells/ml.
f) The solution is distributed into 2-3 large (18 X 150mm) test tubes in approximately 4-6 ml amount
g) The transmittance of the solution at 625nm was between 0.08-0.1 and is standard.
III. Inoculation of Test Plates
I. Optimally within 15minutes after adjusting the turbidity of the inoculum suspension, a sterile cotton swab is dipped into the suspension. The swab was rotated several times and pressed firmly against and inside wall of the test tube above the fluid level. This removed excess fluid from the swab.
II. The dried surface of a sterile agar plate was inoculated by evenly streaking the swab over the entire agar surface. This procedure was repeated by streaking two more times rotating the place approximately 60 °C each time to ensure an even distribution of inoculum. As a final step, the rim of the agar was swabbed.
III. The lid was left ajar for 3-5 minutes, but no more than 15minutes to allow for any excess surface moisture to be absorbed before applying the disks.
Application of Antifungal and Testing Disks to Inoculated Agar Plates
a. Antimicrobial disks and test disks were dispensed onto the surface of the inoculated agar plate. Each disk was pressed down to ensure its complete contact with the agar surface. The disks were placed individually and distributed evenly so that they are no closer than 24 mm from the center to center. Precaution was made that the disk should not move once it had come into contact with the agar surface as the drug / chemical diffuse almost instantaneously.
b. The plates were inverted and placed in an incubator set to 37 °C (±2 °C) with in 15 minutes after the disks were applied.
Results
Each plate was examined after 24 hours of incubation. The resulting inhibition zones were uniformly circular and as streaking was satisfactorily done there was a semi confluent lawn of growth of the Candida species.
The plate was held a few inches above a dark non-luminated background illuminated with reflected light. The inhibition zone diameter was measured, the nearest whole millimeters at the point at which there is a prominent reduction in growth. This is highly subjective and experience results in greater accuracy (trueness) pinpoint micro colonies at the zone edge or local colonies within a zone are encountered frequently and should be ignored. The compounds of concentration 0.5mg/disk marked on Petri plates are as under:
I. Neem oil
II. Pongamia oil
III. Copper Neem soap (CN)
IV. Copper Pongamia soap (CP)
V. Copper Neem -2-amino-6-methyl benzothiazole complex (CNB)
VI. Copper Pongamia-2-amino-6-methyl benzothiazole complex (CPB)
The results are expressed as diameter of inhibition zones of testing disks (and compared with the inhibition zone diameter of the standards antimicrobial agents.
Interpretation of Disk Diffusion Test Results
The strain is susceptible (s) which implies that an infection due to the strain may be appropriately treated with the dose of antimicrobial agent recommended for that type of infection and infecting species, unless otherwise contraindicated. The inhibition zones of test disks were compared with the inhibition zones of antimicrobial disks taken as standard. When the concentrations of the agent/chemical cannot inhibit the strain than the stain in said to be Resistant (R).
Though the Neem and Pongamia oils and Copper soaps are good antifungal agents they show lesser activity than the Copper soap complexes of benzothiazole for Candida species, which suggests that these soap complexes of benzothiazole are more powerful antifungal agents. The presence of benzothiazole and other N, S etc. containing compounds are able to enhance the performance of copper soaps. The enhanced activity of complexes could possibly be explained on the basis of presence of donor atoms N and S as well as the structural compatibility with molecular nature of the toxic moiety.
The studies suggest that lower concentration (0.01mg/disk) and (0.1mg/disk) as well as higher concentrations (0.5mg/disk) of Neem oil, Pongamia oil, Copper (II) Neem soap and Copper (II) Pongamia soap do not show any inhibition zone i.e. the strains are resistant to them, at this concentration while Copper(II) Neem benzothiazole and Copper(II) Pongamia benzothiazole Complexes (Figure 1) at higher concentration showed inhibition zone resulting in the susceptibility of strains (Candida albicans and Candida krusei). It is suggestive that Candida species can be more susceptible with increase in concentration of Copper (II) Neem and Copper (II) Pongamia benzothiazole complexes. Their affectivity increases with their concentration. Thus it is evident that concentration plays a vital role in increasing the degree of inhibition. Fungicidal screening data revealed that at lower concentration the inhibition of growth is less as compared to the higher concentration of the complexes. And so the field is still open for further research.
The enhanced activity of newly synthesized complexes as compared to those of the ligand can possibly be explained on the basis of chelate formation, presence of donor atoms, basicity as well as the structural compatibility with molecular nature of the toxic moiety. Enhanced biological activity of complexes is in accordance with the chelation theory [18]. Suggesting that complexes are more effectively playing their role to check the growth of fungi (in the ternary system: complex + methanol + benzene these types of results have been reported earlier). Some recent studies about antimicrobial activities of newly synthesize 1, 4-benzothiazines possessing thiazolyl / imidazolyl moieties also support our studies [19].
Antifungal testing of Alternaria alternate
The general laboratory techniques followed in the course of this investigation are as [20].
I. Sterilization of Glass-wares: Glassware of Pyrex brand viz. test tubes, conical flasks, pipettes (micro and macro), glass rods and Petri-dishes were thoroughly washed after rinsing with chromic acid each time before sampling and were then sterilized in hot air oven at 160 °C for 24 hours before use.
II. Culture Media Used: The culture media used for the growth of the organism of the present study was P.D.A. The following media were used in the present study38.
a. Potatoes - 200gm
b. Dextrose - 20gm
c. Agar - 200gm
d. Distilled Water - 1000ml
200gm of potatoes were cleaned, cut into pieces and boiled in about 1000ml of water of 2hours. Then the contents were strained using muslin cloth. To this extract 20gm of dextrose, 20gm of agar were added in the flask, before sterilizing the medium.
III. Preparation of Sample solutions: The calculated amount of the testing material {Neem and Karanj oil (extracted from Kernels) & Copper (II) Neem soap and Copper (II) Karanj soap and their complexes} were weighed in a standard flask and the solutions of different concentration in benzene of varying composition were prepared.
IV. Test organism: The test organism used in the study i.e. Alternaria alternata40was also isolated from its natural habitat, purified, characterized & identified. Serial dilution method was used.
V. Fungicidal testing: Firstly 2mL of the fungal broth was poured in sterilized Petri-plates and than the sterilized P.D.A. media was poured and after the gel was set, it was dried with the lids ajar until moisture has evaporated i.e. the surface of agar was kept moist but with no droplets. Into these plates 5 ml of the diluted testing solution was aseptically transferred by rotating the Petri-plates in clockwise and anti clock wise direction 3-4 times and was allowed incubate at 25+10 °C for 24 and 48hours. After the period of incubation the plates were observed for the growth of fungus in different concentration of the testing solution used in the present study.
The data were statistically analysed according to the following formula.
% inhibition = (C-T)/C * 100
C = number of fungal colonies in control plate.
T = number of fungal colonies, in test plate.
Results and Discussion
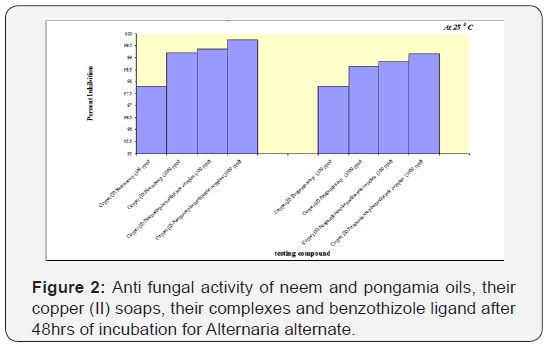
Each plate was examined after 24 and 48 hours of incubation. The number of fungal colonies were counted using colony counter. The % inhibition was calculated according to the given formula above [21,22]. Complexes are better antifungal agents than soaps and Neem complex is more effective than Pongamia complex (Figure 2). The enhanced activity of synthesized complexes as compared to those of the soap can be seen and possibly be explained on the basis of chelate formation, presence of donor atoms, basicity as well as the structural compatibility with molecular nature of the toxic moiety. Enhanced biological activity of complexes is in accordance with the chelation theory [23-25]. It is evident from the results that concentration plays a vital role in increasing the degree of inhibition. Fungicidal screening data revealed that at lower concentration the inhibition of growth is less as compared to the higher concentration of the complexes [26,27]. And so the field is still open for further research and analysis can be made for longer period of incubation and the work can be extended. Earlier workers suggested that complexes are more effectively playing their role to check the growth of fungi [28].
Conclusion
The studies suggest that lower concentration (0.01mg/disk) and (0.1mg/disk) as well as higher concentrations (0.5mg/ disk) of Neem oil, Pongamia oil, Copper (II) Neem soap and Copper (II) Pongamia soap do not show any zone i.e. the strains are resistant to them at this concentration while Copper (II) Neem benzothiazole and Copper (II) Pongamia benzothiazole complexes at higher concentration showed zone resulting in the susceptibility of strains (Candida albicans and Candida krusei). It is suggestive that Candida species can be more susceptible with increase in concentration of Copper (II) Neem and Copper (II) Pongamia benzothiazole complexes. Serial dilution method was used, using PDA as culture media. The result suggests that complexes are better antifungal agents than soaps and Neem complex is more effective than Pongamia complex.
References
- Rai BK, Kumar A (2013) Synthesis, characterization and biocidal activity of some some schiff base and itsmetal complexes of Co(II), Cu(II) and Ni(II). Orient J Chem 29(3): 1187-1191.
- Fugui MB, Ndahi NP, Paul BB, Mustapha AN (2013) Synthesis, characterization, and antimicrobial studies of some vanillin schiff base metal (II) complexes. J Chem Pharm Res 5(4): 22-28.
- Mathur N (2014) Biological Activities of Some New Environmentally Safe 2- Aminobenzothiazole Complexes of Copper (II) Derived Under Microwave Irradiation. Int Arch App Sci Technol 5(1): 37-42.
- Shiekh RA, Rahman IA, Malik MA, Luddin N, and Masudi SM, et al. (2013) Transition metalcomplexes with mixed nitrogen-sulphur (N-S) donor macrocyclic schiff base ligand: synthesis, spectral, electrochemical and antimicrobial studies. Int J Electrochem Sci 8(1): 6972- 6987.
- Choudhary A, Sharma R, Nagar M (2011) Synthesis, characterization and antimicrobial activity of mixedligand complexes of Co (II) and Cu (II) with N, O/S donor ligands and amino acids. Int Res J Pharm Pharmacol 1(6): 172-187.
- Jevtovic V, Ivkovic S, Kaisarevic S, Kovacevic R (2010) Anticancer activity of new copper(II) complexes incorporating, a pyridoxalsemicarbazone ligand. Contemporary Materials 1(2): 133-137 .
- Yadav PS, Devprakash, Senthilkumar GP (2011) Benzothiazole different methods of synthesis and diverse biological activities. International Journal of Pharmaceutical Sciences and Drug Research 3(1): 1-07.
- Sharma R, Saxena M, Sharma N (2012) Synthesis, spectroscopic and fungicidal studies of copper soaps derived from mustard and soyabean oils and their urea complexes. Int J Chem Sci 10(1): 143-149.
- Angelusiu M V, Almajan GL, Ilies DC, Rosu T, and Negoiu M (2008) Cu(II) complexes with nitrogen-oxygen donor ligands: synthesis and biological activity. Chem Bull 53(67): 1-2.
- Raman N, Joseph J, Senthil A, Velan K, Pothiraj C (2006) Antifungal activities of biorelevant complexes of copper(II) with biosensitive macrocyclic ligands. Myco 34(4): 214-218.
- Borhade SS (2012) Synthesis, characterization and antimicrobial activity of copper (II) with 2-chloroquinoline-3-carbaldehyde thiosemicarbazide {1-((2-chloroquinolin-3-yl)methylene) thiosemicarbazide (2-chloro- QAT). Int J Pharm and Life Sci 3(1): 1344-1350.
- Tihile MS, Murade PA (2013) Complexes of 4, 6-dinitrobenzothiazole- 2-amine acetate with some transition metal ions. Journal of Chemical and Pharmaceutical Research 5(2): 5-9.
- Rai BK, Kumar A (2013) Synthesis, Characterization and Biocidal Activity of Some Schiff Base and Its Metal Complexes of Co (II), Cu(II) and Ni(II). Oriental Journal of Chemistry 29(3): 1187-1191.
- Prakasha R, Sharma N, Chaturvedi K (2012) Spectroscopic and antimicrobial studies of mixed ligandcomplexes of transition metal (II) ions with nitro quinoline and dibenzoyl methan. Sci Revs Chem Commun 2(2): 108-114.
- National Committee for Clinical Laboratory Standards (2004) Reference method for antifungal disc diffusion susceptibility testing yeasts propose guideline M44-A, NCCLS, Wayne, PA, USA.
- National Committee for Clinical Laboratory Standards ( 2002) Reference method for antifungal disc diffusion susceptibility testing yeasts propose guideline M27-A2, NCCLS, Wayne, PA, USA
- Bipasa S, Abhijit R (2013) Synthesis, Characterization, Biocidal activity and Phytototoxic effect of Mn(II), Fe(II), Co(II), Cu(II), Zn(II) complexes of 3,5-dinitro benzoic acid. International Journal of Analytical Pharmaceutical and Biomedical Sciences (2): 1-7.
- Mathur N (2011) Studies of solute-solvent interactions and applications of green and blue complexes of copper (II) palmitate with 2-amino benzothiazoles. J Curr Chem Pharm Sc 1(1): 37-51.
- Chandra S, Ballabh P (2013) Synthesis, characterization and physicochemical studies of Ni(II) and Cu(II)complexes with some nitrogen- oxygen and nitrogen sulphur donor ligands. Int J Pharm Sci Res 4(6): 2393-2399.
- Joseph J, Boomadevi JG (2014) Synthesis, structural characterization and biological studies of copper complexes with 2-amino benzothiazole derivatives. Journal of Material and Environment Science 5(3): 693- 704.
- Olagboye SA, Hassan GF (2013) Synthesis, Characterization and Biocidal Evaluation of Azole based Ligands metal Complexes. International Journal of Applied Science and Biotechnology 1(4): 258- 265.
- Mathur N, Heda LC, Mathur VK, Saxena P (2011) Study of CLSI-M44-A Disk Diffusion method for determining the susceptibility of candida species against novel complexes derived from copper stearate with 2-amino benzothiazoles. Tenside Surfactant Detergents 48(1): 1-5.
- Heda LC, Mathur N, Saxena P, Ahmed I (2009) Physical Properties of Copper (II) Soap complexes in Binary Solvent Mixture. Asian Journal of Chemistry 21(1): 57-62.
- Sharma R, Saxena M, Acharya S (2012) Volumetric and viscometric studies of copper (II) surfactant derived from four edible oils in methanol-benzene mixture. Journal of Indian Chemical Society 8: 585- 592.
- Mathur N (2011) Studies of Solute-Solvent Interactions and applications of green and blue complexes of Copper (II) Palmitate with 2-Amino Benzothiazole. Journal of Current Chemical and Pharmaceutical Sciences 1(1): 37-51.
- Chandra S, Ballabh P (2013) Synthesis, Characterization and Physicochemical Studies of Ni (II) and Cu (II) Complexes with some nitrogen- oxygen and nitrogen sulphur donor ligands. International Journal of Pharmaceutical Science and Research 4(6): 2393-2399.
- Bauer AW, Perry DM, Kirby WMM (1959) Single disc antibiotic sensitivity testing of Staphylococci. AMA Arch Intern Med 104(2): 208- 216.
- Bauer AW, Kirby WMM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45(4): 493-496.






























