Synthesis of Some Novel Quinazoline Derivatives Having Anti-Cancer Activity
Arunachalam Sumathy1* and Sivanandy Palanisamy2
1Department of Pharmaceutical Chemistry, Prime College of Pharmacy, India
2Department of Pharmacy Practice, International Medical University, Malaysia
Submission: June 19, 2017; Published: July 21, 2017
*Corresponding author: Arunachalam Sumathy, Department of Pharmaceutical Chemistry, Prime College of Pharmacy, India; Email: sivapalanisamy@yahoo.co.in
How to cite this article: Arunachalam S, Sivanandy P. Synthesis of Some Novel Quinazoline Derivatives Having Anti-Cancer Activity. Glob J Pharmaceu Sci. 2017; 3(2): 555610. DOI: 10.19080/GJPPS.2017.03.555610
Abstract
Cancers are the leading causes of morbidity and mortality worldwide. There are an estimated 14.1 million new cases of cancer in the world and the childhood cancers represent a small fraction of total cancers with the estimated values of 150,000 diagnosis each year. Cancers are a large family of diseases that involve abnormal cell growth with the potential to invade or spread to other parts of the body. Hence, some new semi carbazides containing quinazoline moieties have been synthesized and their ability to inhibit growth of human cancer cell lines has been evaluated. The compound S1 and S3 has shown remarkable anticancer activity. The structures of all the compounds have been confirmed by FT-IR, NMR, and mass spectral analysis.
Keywords: Semicarbazides; Quinazoline; Anticancer activity; Cancer; Morbidity
Abbreviations: NCCS: National Center for Cell Science; FBS: Fetal Bovine Serum; DMEM: Dulbeccos Modified Eagles Medium; TLC: Thin Layer Chromatography; H1NMR: H1Nuclear Magnetic Resonance; UV: Ultra Violet
Introduction
Cancer is the uncontrolled growth and spread of cells. It can affect any part of the body. The growths often invade surrounding tissue and can metastasize to distant sites [1]. Majority of the cancer (90%-95%) are caused by frequent use of tobacco, obesity or over weight, chronic infections and prolonged exposure to radiations and a notable percentage (5%-10%) are due to heredity. Most cancer can be treated by radiology, chemo-therapy and surgery [2]. According to WHO, in 2012 approximately 14 million new cases and 8.2 million cancer related deaths worldwide. The number of new cases is projected to upsurge by about 70% over the next 2 decades. Among men, the most common sites of cancer diagnosed in 2012 were lung, prostate, colorectum, stomach and liver cancer; among women the most common sites diagnosed were breast, colorectum, lung, cervix and stomach cancer.
Tobacco use is the most important cause for around 20% of global cancer deaths and around 70% of global lung cancer deaths. Viral infections caused by HBV/HCV and or HPV are responsible for around 20% of cancer deaths in low- and middle-income countries. Over and above 60% of world’s total new annual cases occur in Africa, Asia and Central and South America, these regions account for 70% of the world’s cancer deaths. The annual cancer cases expected to rise from 14 million in 2012 to 22 within the next 2 decades [3].
Material and methods
General scheme [4]
a) 2-aryl-3-amino-4(3H) quinazolinone
b) 1-(4-oxo-2-arylquinazolin-3(4H)-yl) urea
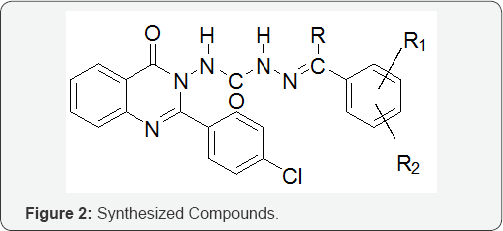
c) 1-(4-oxo-2-arylquinazolin-3(4H)-yl) semicarbazide (Figure 1 & 2)
General procedures
Synthesis of 2-aryl-3-amino-4(3H) quinazolinone from anthranilic acid: Anthranilic acid (0.1mol, 13.71gm) was dissolved in 30ml of dry pyridine by stirring slowly at room temperature. The solution was cooled to 0 °C and a solution of an aromatic acid chloride (4-Chlorobenzoyl chloride) (0.02mole) in 30ml of dry pyridine was added slowly with constant stirring [4,5]. After this addition the reaction mixture was further stirred for half an hour at room temperature and set aside for 1h. The pasty mass obtained was diluted with 50ml of water and washed with water. The crude benzoxazine obtained was dried treated with aqueous sodium bicarbonate solution. When the and recrystallized from diluted ethanol [4-6].
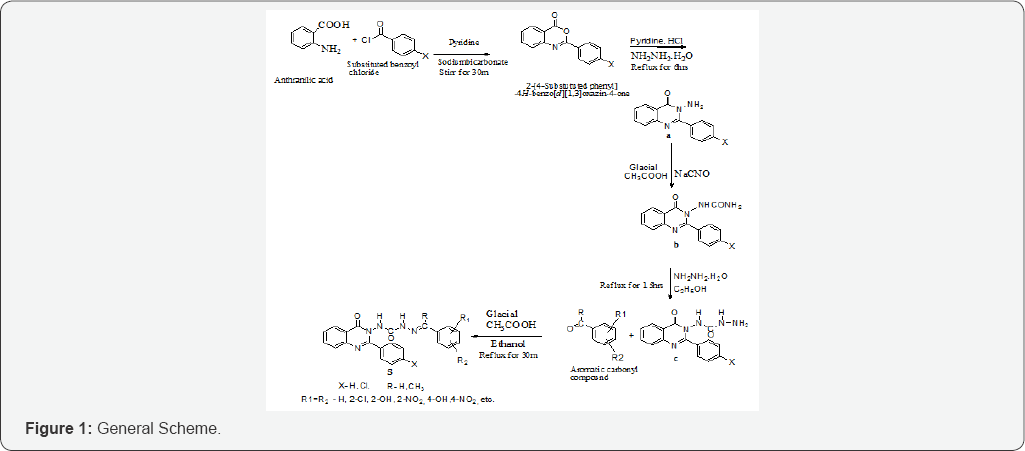
To a cold solution of 2-[4-Chloro phenyl]-4H-benzo[d][1,3] oxazin-4-one(0.05mol) in anhydrous pyridine, (20ml) added a solution of hydrazine hydrate (0.1mol) in anhydrous pyridine (25ml) drop wise with constant stirring. When the addition was complete, the reaction mixture was stirred vigorously for 30min at room temperature and subsequently heated under reflux for 6h under anhydrous reaction conditions. The reaction mixture was allowed to cool at room temperature and poured into ice cold water containing dilute hydrochloric acid. On standing for 1h solidification occurred which was allowed to settle down. It was filtered off, washed repeatedly with water and dried, recrystallized from dilute ethanol [4,6].
Synthesis of 1-(4-oxo-2-arylquinazolm-3(4H)-yl) urea from 2-aryl-3-amino-4(3H) quinazolinone: 3-Amino-2-(4- chlorophenyl)-4(3H) quinazolinone (0.1 mole) was dissolved in 10-30ml of glacial acetic acid and diluted to 100ml with water To this equimolar quantity of sodium cyanate (0.1 mol, 6.5gm) in 50ml of warm water was added with constant stirring. The solution was allowed to stand for 30min. and then cooled in ice for further 30min. The precipitate obtained was filtered with suction, washed with water and dried. The precipitate was recrystallized from boiling water and ethanol mixture [5,6].
Synthesis of 1-(4-oxo-2-arylquinazolin-3(4H)-yl) semicarbazide from 1-(4-oxo-2-arylquinazolm-3(4H)-yl) urea: To a solution of 1-(4-oxo-2-(4-chloro phenyl) -quinazolin- 3(4h)-yl) urea (0.1 mole) in 200ml of water, an equimolar quantity of hydrazine hydrate (0.1mol, 5gm) was added. The reaction mixture was made alkaline by adding 4gm of sodium hydroxide and added 30ml of ethanol to get a clear solution. The reaction mixture was refluxed for 3h with stirring. Excess of ethanol was distilled off under vacuum and then poured in to ice. The obtained precipitate was filtered and recrystallized from 95% ethanol [4,7].
Synthesis of 1-(4-oxo-2-arylquinazolin-3(4H)-yl) aryl semicarbazones from 1-(4-oxo-2-arylquinazolin-3(4H)- yl) semicarbazide: 1-(4-oxo-2-arylquinazolin-3(4H)-yl) semicarbazide (0.01mol) was dissolved in ethanol (20ml) and added slowly to an ethanolic solution of aromatic carbonyl compound (0.01mole). The reaction mixture was acidified with 5ml of glacial acetic acid and refluxed for half an hour. The precipitate obtained was filtered and washed with the mixture of ether and water and dried. The product obtained was recrystallized from 95% ethanol [4-8].
In-Vitro anticancer screening
The human cervical cancer cell line (HeLa), NIH 3T3 mouse embryonic fibroblasts were obtained from National Center for cell science (NCCS), Pune. The HeLa cells were grown in Eagles Minimum Essential Medium containing 10% fetal bovine serum (FBS) and NIH 3T3 fibroblasts were grown in Dulbeccos Modified Eagles Medium(DMEM) containing 10%FBS [8-10]. For the screening experiment, the cells were seeded into 96-well plates in 100|il of respective medium containing 10% FBS , at a plating density of 10,000 cells/well and incubated at 37 °C, 5%CO2, 95% air and 100% relative humidity for 24hrs prior to addition of samples. The extracts were solubilised in DMSO and diluted in respective medium containing 1%FBS. After 24h, the medium was replaced with the respective medium containing the samples at various concentrations and incubated at 37 °C, 5% CO2, 95% air and 100% relative humidity for 48h.
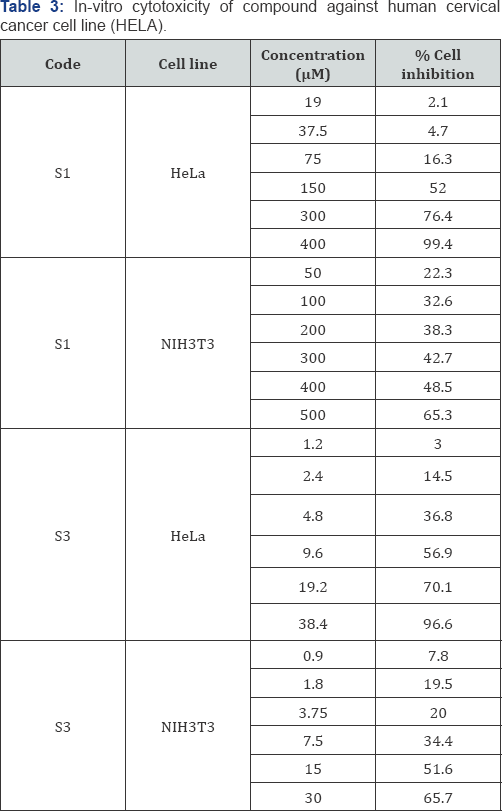
Triplicate was maintained and the medium without samples were served as control [8,9]. After 48h, 10μl of MTT (5mg/ml) in phosphate buffered saline was added to each well and incubated at 37 °C for 4h. The medium with MTT was then flicked off and the formed crystals were solubilised in 100 μl of DMSO and measured the absorbance at 570nm using microplate reader [10]. The percentage cell inhibition and IC50 were calculated and are given in Table 1.
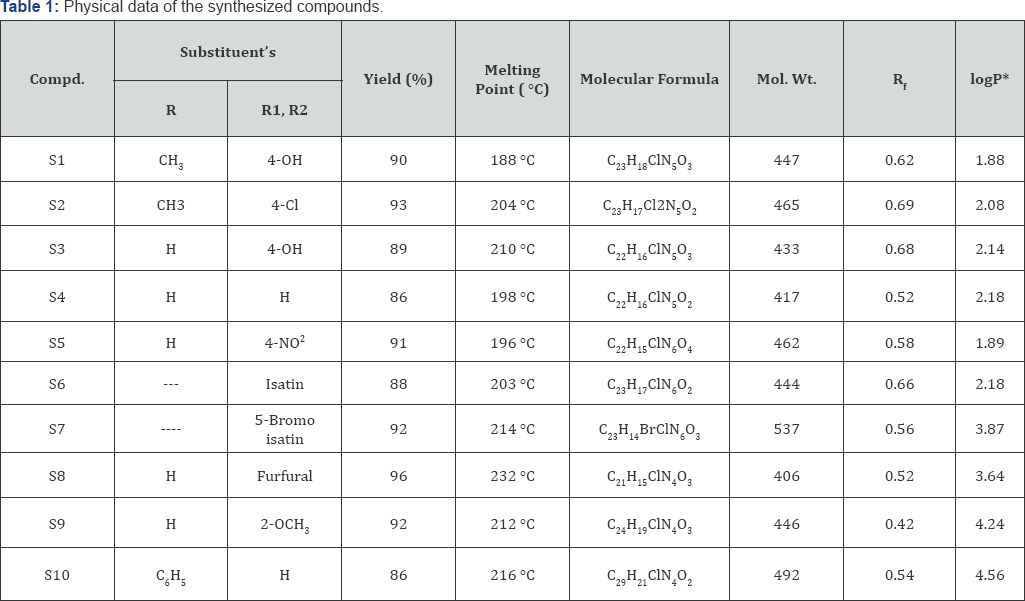
*Log P was calculated by partition coefficient determination using Octanol and buffer system.
Rf- Solvent system- ethyl acetate-butanol:water (6:3:1)
Results and Discussion
The compounds were synthesized by various reaction intermediate such as:
• Synthesis of 2-(4-chloro phenyl)- 4H-3, 1- Benzoxazin-4 one through N-Benzoylation followed
• dehydrative cyclisation mechanism.
• Synthesis of 2-(4-chloro phenyl)-3-amino-4(3H)- Quinazoline.
• Synthesis of 2-(4-chloro phenyl)-3-amino-4(3H)- Quinazoline urea.
• Synthesis of 2-(4-chloro phenyl)-3-amino-4(3H)- Quinazoline semicarbazide.
• Semicarbazones reactions of 2-(4-chloro phenyl)-3- amino-4(3H)-Quinazoline semicarbazide and aromatic aldehydes and ketones.
The synthesized compounds purity and its structural elucidation were done by various analytical techniques such as Melting point, Thin Layer Chromatography (TLC), Ultra Violet Spectroscopy (UV), Infrared Spectra (IR) and H1 Nuclear magnetic resonance (H1NMR).
Melting point
The melting point is an important physical property of a compound and it can be used to identify a substance and its purity. The melting point of solid is the temperature at which the solid exists in equilibrium with its liquid under an external pressure of one atmosphere [11]. A synthesized compounds melting point and its reactants melting point were recorded by open capillary tube method [4,12], which are uncorrected. A reactant and products melting point were differing from each other. It clearly indicates that the formation of a new chemical entities. The melting point values of the synthesized compounds are presented in Table 2.
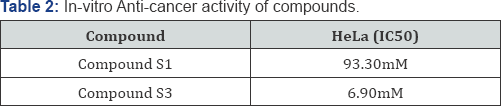
Data are the mean of three or more experiment and are reported as mean±standard error of the mean (SEM).
Thin layer chromatography
Thin layer chromatography (TLC) is a very expedient and effective technique for the separation and identification of inorganic ions. It permits selective separations, simple detection and easy manipulation of the mobile phase. As a result, numerous sorbents and even better number of mobile phases have been developed for attaining the improved chromatographic performance in terms of selectivity, resolution, rapidity and reproducibility [13]. Thin-layer chromatography can be used to monitor the progress of a reaction, identify compounds present in a given mixture, and determine the purity of a substance [14]. Thin layer chromatography techniques were performed for all synthesized compound as well as the parent compounds, all synthesized compounds gave a single spot whose Rf values are different from their reactants. It ultimately shows that the compound's purity and completion of the reaction. The Rf value are presented in Table 3.
Infra-red spectra
Infrared spectroscopy is one of the classical methods for structure determination of small molecules. This standing is due to its sensitivity to the chemical composition and architecture of molecules [15]. Infra-Red spectroscopy was taken for all the synthesized compounds. The characteristic absorption peaks were observed for all relevant groups. Aromatic acid chlorides strongly absorb at 1800-1770cm-1 which was not observed in spectrum, it clearly gives a chemical entity in step I. The absorption peaks around 1600-1500cm-1 indicates that the formation of C=N Schiff bases. C=O stretching vibration around 1870cm-1 was not appeared in spectrum. It shows that there was no impurities like aldehyde/ketone., C-C, C=C & C=N ring stretching vibration at 1050-1200,754cm-1 also appeared and aromatic amide N-H was observer at 3100-3500cm-1 and all other relevant groups absorption were observed for all the synthesized compounds.
H1 Nuclear magnetic resonance
NMR or nuclear magnetic resonance spectroscopy is a technique used to determine a compound's unique structure. It identifies the carbon-hydrogen framework of an organic compound. Using this method and other instrumental methods including infrared and mass spectrometry, scientists are able to determine the entire structure of a molecule [16]. 1H nuclear magnetic spectra were taken for all the synthesized compounds. Aromatic protons were observed 6.68-8.135ppm, Amide N-H proton was observed at 6.05-6.405ppm, for all the synthesized compounds.
Anti-cancer activity
Cancer is a multi-step disease incorporating physical, environmental, metabolic, chemical and genetic factors, which play a direct and/or indirect role in the induction and deterioration of cancers [17]. Cancer has a major impact on society in the United States and across the world. Cancer statistics describe what happens in large groups of people and provide a picture in time of the burden of cancer on society [18]. Hence, some of the synthesized compounds were tested for in-vitro anticancer activity. Among the synthesized derivatives compound S1 and S3 were screened for in-vitro cytotoxicity effect. The results indicate that the synthesized compound possesses cytotoxicity against both cancer cells as well as normal cells. It proves that suitable structural modifications will have to be carried out to get novel compounds having potent anticancer activity with least effect on normal cells [19-21].
Conclusion
In summary, a new series of quinazoline derivatives (s1-s10) have been synthesized and evaluated for anti-cancer as well as anti-bacterial activities. Among the synthesized compounds S1, S3 were the potent compounds with good results for anti-cancer activity. These quinazoline derivatives S1, S3 represent promising structures for the development of new compounds with better activity results and for future in-vivo analysis.
References
- Denny WA, Rewcastle GW, Bridges AJ, Fry DW, Kraker AJ (1996) Structure-activity relationships for 4-anilinoquinazolines as potent inhibitors at the ATP binding site of the epidermal growth factor receptor in vitro. Clin Exp Pharmacol Physiol 23(5): 424-427.
- Latli B, Wood E, Casida JE (1996) Insecticidal quinazoline derivatives with (trifluoromethyl) diazirinyl and azido substituents as NADH: ubiquinone oxidoreductase inhibitors and candidate photoaffinity probes. Chem Res Toxicol 9(2): 445-450.
- World Cancer Report (2014) World Health Organisation-Cancer. Geneva, Switzerland.
- Sumathy A, Palanisamy S, Karthik D (2013) A study on synthesis, molecular properties and antimicrobial activity of some quinazoline derivatives. J Chem Pharm Res 5(10): 1-9.
- Kumar P, Agarwal JC, Nath C, Bhargava KP, Shanker K (1981) Substituted dopamine quinazolones as potent antiparkinsonian agents. Pharmazie 36(11): 780.
- Brana MF, Castellano JM, Keihauer G, Machuca A, Martin Y, et al. (1994) Benzimidazo [1,2-c]quinazolines: a new class of antitumor compounds. Anticancer Drug Des 9(6): 527-538.
- GoodMan LS, Gilman A (2011) The Pharmacological Basis of Therapeutics. (12th edn) McGraw Hill New York.
- Boyle FT, Matusiak ZS, Hughes LR, Slater AM, Stephens TC, et al. (1993) Substituted-2-desamino-2-methyl-quinazolinones. A series of novel anti-tumour agents. Adv Exp Med Biol 338: 585-588.
- Rosowsky A, Hynes JB, Queener SF (1995) Structure-activity and structure-selectivity studies on diaminoquinazolines and other inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. Antimicrob Agents Chemother 39(1): 79-86
- Vacca A, Bruno M, Boccarelli A, Coluccia M, Ribatti D, et al. (2002) Inhibition of endothelial cell functions and of angiogenesis by the metastasis inhibitor NAMI-A. Br J Cancer 86(6): 993-998.
- Melting Point of an Organic Compound.
- Collins L, Franzblau SG (1997) Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 41(5): 1004-1009.
- Dhote SS, Deshmukh L, Paliwal L (2012) Separation and Identification of Heavy Metal Ions by Thin Layer Chromatography on Stannous Silicate Layer. Chem Sci J 62: 1-11.
- Reich EA (2007) High-performance thin-layer chromatography for the analysis of medicinal plants. (Illustrated edn.)New York: Thieme.
- Demirdoven N, Cheatum CM, Chung HS, Khalil M, Knoester J, et al. (2004) Two-dimensional infrared spectroscopy of antiparallel beta-sheet secondary structure. J Am Chem Soc 126(25): 7981-7990.
- http://www.chem.ucla.edu/~harding/notes/notes_14C_nmr02.pdf
- Rahman S, Salehin F, Iqbal A (2011) In-vitro antioxidant and anticancer activity of young Zingiber officinale against human breast carcinoma cell lines. BMC Complementary and Alternative Medicine 11:76.
- NCI (2017) Cancer Statistics. National Cancer institute, USA.
- Schleiss M, Eickhoff J, Auerochs S, Leis M, Abele S, et al. (2008) Protein kinase inhibitors of the quinazoline class exert anti-cytomegaloviral activity in-vitro and in-vivo. Antiviral Res 79(1): 49-61.
- Narla RK, Liu XP, Myers DE, Uckun FM (1998) 4-(3'-Bromo- 4'hydroxylphenyl)-amino-6,7-dimethoxyquinazoline: a novel quinazoline derivative with potent cytotoxic activity against human glioblastoma cells. Clin Cancer Res 4(6): 1405-1414.
- Kovala DD, Boccarelli A, Demertzis MA, Coluccia M (2007) In-vitro antitumor activity of 2-acetyl pyridine 4n-ethyl thiosemicarbazone and its platinum(II) and palladium(II) complexes. Chemother 53(2): 148-152.






























