Solid Dispersion - a Novel Approach for Enhancement of Bioavailability of Poorly Soluble Drugs in Oral Drug Delivery System
Singh N and Sarangi Mk*
Sardar Bhagwan Singh Post Graduate Institute of Biomedical Sciences and Research, India
Submission: May 6, 2017; Published: July 11, 2017
*Corresponding author: Manoj Kumar Sarangi,Sardar Bhagwan Singh Post Graduate Institute of Biomedical Sciences and Research, India; Email: manojsarangi@rediffmail.com
How to cite this article: Singh N, Sarangi M. Solid Dispersion - a Novel Approach for Enhancement of Bioavailability of Poorly Soluble Drugs in Oral Drug Delivery System. Glob J Pharmaceu Sci. 2017; 3(2): 555608. DOI: 10.19080/GJPPS.2017.03.555608
Abstract
Improving oral bioavailability of drugs those given as solid dosage forms remains a challenge for the formulation scientists due to solubility problems. The dissolution rate could be the rate-limiting process in the absorption of a drug from a solid dosage form of relatively insoluble drugs. Therefore increase in dissolution of poorly soluble drugs by solid dispersion technique presents a challenge to the formulation scientists. Solid dispersion techniques have attracted considerable interest of improving the dissolution rate of highly lipophilic drugs thereby improving their bioavailability by reducing drug particle size, improving wettability and forming amorphous particles. The term solid dispersion is dealing with a group of solid products which is consisting of at least two different components, generally a hydrophilic inert carrier or matrix and a hydrophobic drug. This article reviews historical background of solid dispersion technology, limitations, classification and various preparation techniques with its advantages and disadvantages. This review also discusses the recent advances in the field of solid dispersion technology. Based on the existing results and authors’ reflection, this review give rise to reasoning and suggested choices of carrier or matrix and solid dispersion procedure.
Keywords: Carrier; Dissolution; Matrix; Poorly soluble drug; Solid dispersion; Solubility enhancement
Abbreviations: SMEDDS: Self-Micro Emulsifying Drug Delivery Systems; G: Energy; GI: Gastrointestinal; PVP: Pyrolidone; PEG: Polyethylene Glycols; HPMC: Hydroxyl Propyl Methyl-Cellulose; HPbCD: Hydroxypropyl-b-Cyclodextrin; NAP: Solubility of Naproxen; AMP: Ampelopsin; BCD: β-Cyclodextrin; HPBCD: Hydroxyl Propyl-β-Cyclodextrin; SDs: Solid Dispersions; GBM: Glibenclamide; PEG: Polyethylene Glycol; SDs: Solid Dispersions; KT: ketoconazole; ME: Microemulsion; HPMCAS: Methylcellulose Acetate Succinate; IND: Indomethacin; PEG4000: Polyethylene Glycol 4000; PVP K30: Polyvinyl Pyrollidone
Introduction
It has been estimated that nearly 35-40 % of drugs suffer from poor aqueous solubility and it affects the absorption of drug from gastrointestinal tract that leads to high inter and intra subject variability, poor oral bioavailability, increase in dose, reduction in therapeutic efficiency and finally failure in formulation development. Various formulation strategies like micronization, solubilization, complexation, dendrimers for drug solubilization, formation of solid solutions/dispersions with hydrophilic carriers, self-micro emulsifying drug delivery systems (SMEDDS). Nanoparticulate approaches, spray drying, pro-drug approaches and salt synthesis had been attempted for solubility enhancement. An attractive possibility would be represented by implementing a simple solid dispersion technique by utilizing several hydrophilic carriers. Such technique impart a means of reducing particle size to a nearly molecular level, presenting a variety of processing and excipients options which allow for flaccidity when formulating oral delivery systems of low water soluble drugs with cost effectiveness and denoting dose reduction [1].
Solubility and dissolution
The solubility behaviour of a drug is a crucial determinant of its oral bioavailability. There have been always certain drugs, for which solubility has conferred a challenge to the development of a suitable formulation for oral administration. With the recent advent of high throughput screening of potential therapeutic agents, the number of poorly soluble drug moieties has increased suddenly and thus the formulation of poorly soluble compounds for oral delivery now presents one of the most frequent and greatest challenges to formulation scientists in the pharmaceutical industry [2]. The free energy (G) is a measure of the energy available to the system to perform work. Its value decreases during a continuously occurring process unless and until an equilibrium position is achieved when no further energy can be made available, i.e., ΔG=0 at equilibrium [3]. The solution was developed when an equilibrium is established between un-dissolved and dissolved solute components in a dissolution process is termed as saturated solution.
The amount of substance that passes into solution in order to establish the equilibrium at constant pressure and temperature and so produced a saturated solution is known as the solubility of the Consideration of the modified Noyes-Whitney equation (1) provides some hints as to how the dissolution rate of even very poorly soluble compounds might be improved to minimize the limitations to oral bioavailability of substance.
dC/dt = AD (Cs - C)/h
Where, dC/dt is the rate of dissolution, A is the surface area available for dissolution, D is the diffusion coefficient of the compound, Cs is the solubility of the compound in a dissolution medium, C is the concentration of drug present in the medium at a time t and h is the thickness of the diffusion boundary layer adjacent to the surface of the dissolving compound [4]. The main possibilities or improving dissolution according to this analysis are to increase the surface area available for dissolution by decreasing the size of the particles present in the solid compound by optimizing the wetting phenomenon of the compound surface, to decrease the boundary layer thickness, to ensure sink conditions for dissolution and, last but not definitely the least, to enhance the apparent solubility of the drug molecules under physiologically relevant conditions. The absorption of drug from the gastrointestinal (GI) tract can be limited by several factors with the most important contributors being poor aqueous solubility and/or poor membrane permeability of the drug molecule.
While delivering an active agent orally, it is very much important that it must dissolve in gastric and/or intestinal fluids before it can reach systemic circulation through GI membrane permeability. Hence, a drug with poor aqueous solubility will exhibit dissolution rate limited absorption, and a drug with poor membrane permeability will basically exhibit permeation rate limited absorption. Thus, two areas of pharmaceutical research that focus on improving the oral bioavailability of the active agents include enhancing solubility and dissolution rate of poorly water-soluble drugs and increasing the permeability of poorly permeable drugs [5].
Several Approaches for enhancement of drug dissolution/ bioavailability of poorly soluble drugs
I. Physical modifications [6]
o Particle size
o Micronization
o Nanosuspensions
o Modifications of the crystal habit
o Polymorphs
o Pseudopolymorphs (including solvates)
o Complexation/solubilization
o Utilization of surfactants
o Utilization of cyclodextrines
o Dispersion of Drug in a carrier
o Implication of Eutectic mixtures
o Solid dispersions (non-molecular)
o Solid solutions
II. Chemical modifications
o Soluble prodrug approach
o Salt formation
Bioavailability is defined as the rate at which the relative amount of an administered dose of a drug reaches to the systemic circulation from its site of administration (American Pharmaceutical Association, 1972). Various factors that influence the bioavaibility of the drug includes the gastric emptying rate, physiochemical properties of the drug, drug formulation type, enzymes induction/inhibition by other drugs/ foods, circadian differences, transporters, diseased state, health of the gastrointestinal tract etc. As the contents of GI tract are aqueous in nature, hence a drug with poor aqueous solubility possesses low saturation solubility which is correlated with a low dissolution rate, resulting a poor oral bioavailability. About 60% of drugs coming directly from synthetic origin have solubility below 0.1mg/ml [7].
Solid dispersion
Solid dispersions are one of the most successful strategies to improve drug release of poorly soluble drugs. Sekiguchi and Obi were the first to describe on solid dispersions in 1961. Solid dispersion is one of the important strategies to tackle dissolution- rate-limited oral absorption of poorly soluble compounds. Formulation of poorly soluble compounds as solid dispersions might lead to particle size reduction, improved wetting, reduced agglomeration, changeability in the physical state of the drug molecules and possibly a dispersion in the molecular level, according to the physical state of the solid dispersion. The term solid dispersion refers to a group of solid products consisting of at least two different components, generally a hydrophilic matrix and a hydrophobic drug. The matrix can be either crystalline or amorphous. The drugs can be dispersed molecularly, either in amorphous particles (clusters) or in crystalline particles.
Solid dispersion
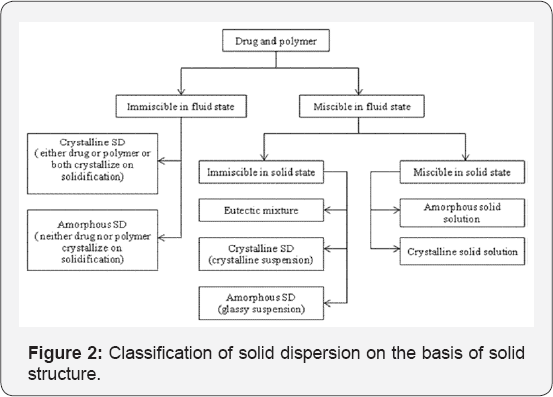
Solid dispersions are classified by various ways, on the basis of their solid state structure as well as on the basis of carrier used. It is relevant to classify various systems of solid dispersion as per as their fast release mechanisms are concerned. Riegelman and Chiou classified solid dispersions into the following six representative types: Simple eutectic mixtures, amorphous precipitations in a crystalline carrier, solid solutions, glass solutions and glass suspensions, compound or complex formation, and combinations of the previous five types [8]. Given below is classification of solid dispersion on the basis of carrier used and solid structure in Figure 1 & 2 respectively.
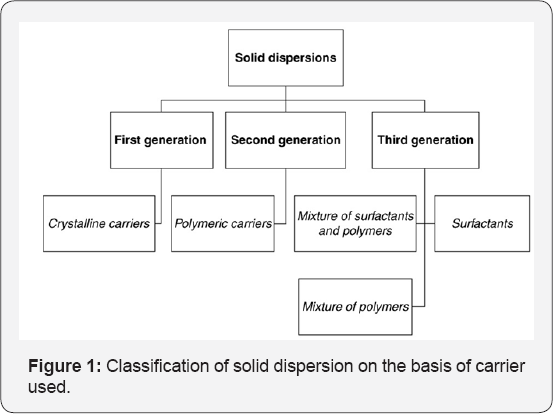
First generation: The first generation solid dispersions can be developed by using crystalline carriers like urea and sugar, which were the first carriers to be imparted in solid dispersion. They are having the disadvantage of forming crystalline solid dispersion, which were thermodynamically more stable and cannot release the drug as quickly as amorphous ones.
Second generation: In second generation solid dispersions, amorphous carriers are used instead of crystalline carriers, which are usually polymers. These polymers include synthetic polymers such as poly vinyl pyrolidone (PVP), polyethylene glycols (PEG), ethyl cellulosepolymethacrylates, natural product based polymers such as hydroxylpropylmethyl-cellulose (HPMC) and hydroxypropyl cellulose or starch derivatives like cyclodextrins.
Third generation: Recently, it has been observed that the dissolution profile can be improved further, if the carrier has surface activity or self-emulsifying properties. Therefore, third generation solid dispersions were developed. The use of surfactant such as inutec SP1, inulin, compritol 888 ATO, gelucire 44/14 and poloxamer 407 as carriers were shown to be effective in originating high polymorphic purity and enhanced in vivo bioavailability.
Drug and polymer exhibiting immiscibility in fluid state
In case a drug and polymer are immiscible in their fluid state, it is expected that they would not exhibit miscibility on solidification of the fluid mixture. Hence such systems might be considered as similar to their corresponding physical mixtures and any short of enhancement in dissolution performance may be leading to modification in morphology of drug and/or polymer due to the physical transformation (i.e., solid to liquid state and back), enhanced surface area and/or intimate drug - polymer mixing.
Drug and polymer exhibiting miscibility in fluid state
If the polymer and drug are miscible in their fluid state, then the mixture may or may not undergo phase separation during solidification, thus influencing the structure of solid dispersion.
Eutectic mixtures
Eutectic mixtures were first described as solid dispersions in 1961 by Sekiguchi & Obi. Eutectic mixtures are formed when the polymer and drug are miscible in their molten state, but on cooling, they crystallize just like two distinct components with negligible miscibility.
Crystalline solid dispersion
A crystalline solid dispersion is developed when the rate of drug crystallizes from drug-polymer miscible mixture is greater than that of the rate at which drug-polymer fluid mixture solidifies.
Amorphous solid dispersion
If the drug-polymer fluid mixture is cooled at a rate that does not allow for drug crystallization, then drug is kinetically trapped in its amorphous or a "solidified-liquid" state. Such types of dispersions have a high risk of potential for conversion to a less soluble and more stable crystalline form.
Solid solution
Solid solution is defined as a solid dispersion which is miscible in its solid as well as fluid state. These solid solutions may be either of amorphous or crystalline type. In case of amorphous solid solutions, since the drug is molecularly dispersed in the carrier matrix, hence its effective surface area is predominantly higher and thus the dissolution rate of that drug is increased. Crystalline solid solution may results when a crystalline drug is trapped within a crystalline polymeric carrier.
o As per as the miscibility of the two components is concerned, the solid solutions are either continuous or discontinuous type. In continuous solid solutions, the two components are miscible in the solid state in all proportions. The components that are miscible at extremes of composition but immiscible at intermediate composition are referred to as discontinuous solid solutions.
o As per the criteria of molecular size of the two components are concerned, the solid solutions are classified as interstitial and substitutional. In the substitutional solid solution, the solute molecule substitutes the solvent molecule in the crystal lattice but, in case of interstitial solid solution, it is obtained when the solute (guest) molecule occupies the interstitial space in the solvent (host) lattice.
Methods of preparation
Various methods for preparation of solid dispersion are given in Figure 3 [9].
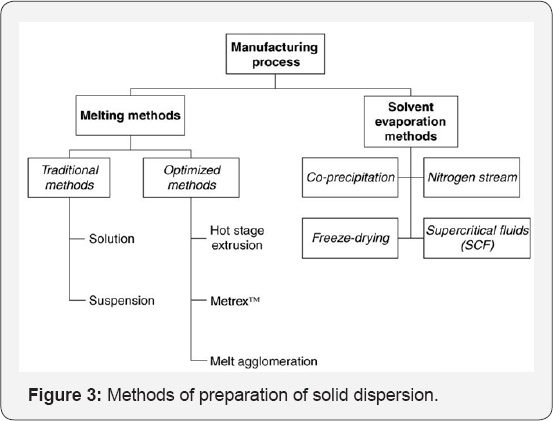
Solvent evaporation method
The solvent evaporation technique mainly aims at dissolving the drug and carrier simultaneously in a common solvent, followed by removal of solvent through evaporation. Identification of a common solvent for both carrier and drug can be problematic and a complete solvent removal from the product can be a lengthy process. The solvent can be removed by various processes including heating of the mixture, vacuum drying, slow evaporation of the solvent at low temperature, the rotary evaporators, freeze drying and spray drying. Many drugs and polymers which could not be utilized for the melting method due to their high melting points could be used for solvent evaporation method.
Melting method
The melting method includes the melting of a physical mixture of carrier and drug into the liquid state followed by cooling until solidification. However, the method is not useful for thermolabile drugs and thus incomplete miscibility is observed between the molten carrier and solid drug.
Melting solvent method
The melting solvent method is a combination of the two methods like melting and solvent evaporation method. It is carried out by dissolving the drug in a suitable solvent and then mixing of the resultant solution with the molten carrier followed by cooling into solidification. The advantage of this method is that it requires lower temperatures with lesser risk of decomposition of thermo labile drugs.
Spray drying process
Spray drying is the process where a solution of drug substance and carrier is evaporated by spraying the solution as fine droplets into a chamber under controlled conditions of heat, humidity and air flow. The medium of drying is mainly associated with hot air and the product is thus separated after completion of drying.
Hot extrusion method
Hot melt extrusion (HME) is a widely used process in plastic, rubber, and food industry. The process has been implimented for the preparation of solid dispersion in a single step. In earlier periods HME is used to prepare solid dispersion to enhance solubility of poorly water soluble drugs. But now a days, its value in developing controlled release dosage forms has gained more attraction. The key advantages of hot-melt extrusion technique include lower temperature and shorter residence time of the drug carrier mixture. Basically, the physical mixture of drug substance along with other ingredient is fed into the heated barrel of extruder at a controlled rate. As the physical mixture is supplied through heated screws, it is converted into a fluid like state, thus allowing an intimate and homogeneous mixing by the high shear of extruder screws. The die helps in shaping the melt in the required form such as pellets, granules, tablets, sticks, sheets or powder.
Supercritical fluid technology
The supercritical fluid endures as a single fluid phase above its critical pressure and temperature. Carbon dioxide is the most commonly used supercritical fluid. In SAS or GAS process a mixture of drug and polymer is sprayed with the help of an atomizer into a chamber filled with supercritical fluids. The extraction and expansion of organic solvent into the compressed gas result in lowering the solvent power of organic solvent for polymer as well as drug, thus leading to their precipitation.
Lyophilization (freeze drying)
An important advantage of freeze drying is that the drug is exposed to a minimal thermal stress condition during the formation of the SDs. However, the most important advantage is that the risk of phase separation is minimized as soon as the solution is vitrified.
Electrostatic spinning method
Electrostatic spinning method includes the introduction of a liquid into an electric field whereas the liquid is used to develop fibers. After withdrawal from the liquid, the fibers harden, which may include mere cooling, chemical hardening or evaporation of solvent, and then hardened fibers may be collected upon a suitably charged surface. Tubular products comprising polyurethane fibers can be prepared by this electrostatic spinning method. One example of such type of tubular product is a vascular prosthesis, basically a synthetic blood vessel.
Advantages of solid dispersion
Particles with reduced particle size: Molecular dispersions, as solid dispersions, represents the last stage of particle size reduction, and thus the drug possess a molecular dispersion in the dissolution medium after the dissolution of its carrier.
Particles with improved wettability: A strong contribution to the enrichment of drug solubility is related to the drug wetability improvement verified in solid dispersions. Carriers with surface activity, such as bile salts and cholic acid, when used, can potentially increase the wetability properties of drugs.
Particles with higher porosity: Particles with solid dispersions have been observed to have a higher degree of porosity. The increased porosity of solid dispersion particles also accelerates the drug release profile.
Drugs in amorphous state: Poorly water soluble crystalline drugs, in their amorphous state tend to have a higher solubility. However, the enhancement of drug release can usually be obtained using the drug moiety in its amorphous state, as no energy is required to break up the crystal lattice in the interim of dissolution process. In case of solid dispersions, drugs are conferred as supersaturated solutions after the system dissolves, and it is speculated that, if drugs precipitate, then it could be converted into metastable polymorphic form with a higher solubility than its most stable crystal form.
Disadvantages
o Carriers with High melting point cannot be used.
o Thermal degradation or instability may result at themelting point.
o Decomposition may take place, often dependent upon composition, fusion time and rate of cooling.
o Sublimation or Evaporation and polymeric transformation of the dispersion component may takes place.
o Solidified melt may be tacky and unhandable.
Carriers
o The properties of the carrier have been the major influence on dissolution characteristics of dispersed drug molecules. However the carrier should meet the following criteria's to be suitable for increasing the dissolution rate of the drug.
o It should be water soluble with intrinsic rapid dissolution properties.
o It should be nontoxic and pharmacologically inert.
o It should be heat stable with low melting point for melt method.
o It must be soluble in variety of solvents for evaporation in solvent method.
o It must be able to increase the aqueous solubility of drug.
o It must be chemically compatible with drug and should not possess a strong complex with it.
o It must stabilize the supersaturated solution formed after dissolution of solid dispersion in GIT
o It must have functional groups which are either acceptors or donors for hydrogen bonds, as specific interactions increase the solid solubility of the drug into its carrier.
o It should have high glass transition temperature.
Characterization of solid dispersion
There are so many methods available for contributing information regarding the physical nature of solid dispersion system [9].
Thermal Analysis Techniques
Thermal analysis comprises a group of techniques in which a physical property of a substance is measured as a function of temperature, while the substance is subjected to a controlled temperature program. In differential thermal analysis, the temperature difference that develops between an inert reference material and the sample is measured, when both of them are subjected to an identical heating condition.
X-ray crystallography
X-ray crystallography is a substantial method for determining the arrangement of atoms within a crystal lattice, in which a beam of X-rays strikes on a crystal and diffracts into many specific directions. A crystallographer can produce a three dimensional picture of the density of electrons within the crystal by analyzing the angles and intensities of these diffracted beams. From this electron density, the mean positions of the atoms in the crystal can be determined, along with their chemical bonds, disorder and various other information.
Spectroscopy
Spectroscopy is defined as the study of the interaction between matter and radiation as a function of wavelength (λ). In fact, historically, spectroscopy is referred to the use of visible light dispersed according to its wavelength, e.g. by a prism. However in later stage the concept was expanded greatly to comprise any measurement of a quantity as a function of either frequency or wavelength. Thus it also can be referred to as a response to an alternating field or varying frequency (v).
Dissolution testing
Dissolution experiments can be carried out in triplicate on the binary as well as ternary dispersions. The tests were performed according to the USP 24 method 2 in a dissolution apparatus. To simulate the dissolution of a weak basic compound in the stomach, 500ml of simulated gastric fluid without pepsin was used as dissolution medium at a temperature of 37 °C and a paddle speed of 100rpm. The amount of the spray-dried powders, equivalent to the drug dose of 100mg, was added to the dissolution medium.
Environmental scanning electron microscopy
The morphology of the spray-dried ternary solid dispersions was characterized with environmental scanning electron microscope, operating at 25Kv of accelerating voltage in association with a vacuum. The samples were sprayed on double-sided carbon tape which was mounted on a conventional SEM stubs.
Review of literature on solid dispersion
Dressman et al. studied on improval of drug solubility for oral delivery by using solid dispersion. They gave an overview of the historical background and definitions of the various systems including eutectic mixtures, solid dispersions and solid solutions. It is concluded that although solid solutions have tremendous potential for improving drug solubility, 40 years of research have resulted in only a few marketed products using this approach. Thus, with an introduction of new manufacturing technologies such as hot melt extrusion, it could be possible to overcome the problems in scale-up.
Mura et al. [10] studied combined effect of hydroxypropyl- b-cyclodextrin (HPbCD) and polyvinylpyrrolidone (PVP) on the solubility of naproxen (NAP). The results of solid state studies accounted for the occurrence of mechanically- and/or thermally- induced stronger interactions in ternary than in binary systems, that in some cases led to a complete loss of NAP crystallinity. Ruan et al. [11] studied solubility of ampelopsin (AMP) in water by two systems: solid dispersions with polyethylene glycol 6000 (PEG 6000) or polyvinylpyrrolidone K-30 (PVP K30) and inclusion complexes with β-cyclodextrin (BCD) and hydroxyl propyl-β-cyclodextrin (HPBCD).It was concluded that improvement of solubility using polymers was in the following order: HPBCD≈BCD> PVP K30 > PEG 6000.
Chauhan et al. [12] prepared solid dispersions (SDs) of glibenclamide (GBM); a poorly water-soluble drug and polyglycolized glycerides (Gelucire®) with the aid of silicon dioxide (Aerosil® 200); as an adsorbent, were prepared by spray drying technique. The study demonstrated the high potential of spray drying technique for obtaining the stable as well as free flowing SDs of poorly water-soluble drugs using polyglycolized glycerides carriers with the aid of silicon dioxide as an adsorbent.
Heo et al. [13] prepared the polyethylene glycol (PEG) 6000-based solid dispersions (SDs), by incorporating various pharmaceutical excipients or microemulsion systems, using a fusion method, to compare the dissolution rates. The ketoconazole (KT), a potent antifungal agent, was selected to be as a model drug. They found that when hydrophilic and lipophilic excipients were combined and incorporated into PEG-based SDs, a remarkable enhancement of the dissolution rate was observed. The PEG-based SDs, incorporating a self microemulsifying drug delivery system (SMEDDS) or microemulsion (ME), were also useful at improving the dissolution rate by forming a microemulsion or dispersible particles within the aqueous medium.
Urbanetz et al. [14] prepared solid dispersions of Nimodipine and polyethylene glycol 2000. It was found that the absence of crystalline drug material in solid dispersions containing Nimodipine and polyethylene glycol 2000 is the prerequisite for a high dissolution rate and a remarkable super saturation in the dissolution medium. Thus, shock freezing during the preparation process, along with low storage temperatures and low relative humidities are useful to prevent recrystallisation.
Costa et al. [4] thoughly studied on solid dispersion as a strategy to improve oral bioavaibility of poorly water soluble drugs. They have disclosed the recent advances related to the area of solid dispersions. It was found that by reducing drug particle size to the absolute minimum, and hence improving drug wettability, bioavailability may be significantly improved. Ansari et al. [15] studied the physicochemical characteristics of polyvinyl pyrrolidone, dihydroartemisinin and their solid dispersions which were evaluated at various proportions of drug and polyvinylpyrrolidone. It was found that dihydroartemisinin became more amorphous as drug carrier ratio was enhanced in solid dispersions.
Konno et al. [16] studied the effect of polymer type on the dissolution profile of amorphous solid dispersions containingFelodipine. In the current study, the dissolution profiles of solid dispersions of Felodipine formulated with hydroxypropyl methylcellulose (HPMC), poly(vinylpyrrolidone) (PVP), or hydroxypropyl methylcellulose acetate succinate (HPMCAS) were compared. However HPMCAS was found to maintain the highest level of super saturation for the greatest length of time for both the dissolution and solution crystallization experiments, whereas PVP was observed to be the least effective crystallization inhibitor. It was concluded that all polymers appeared to reduce the crystal growth rates of Felodipine at an equivalent super saturation and this mechanism most likely contributes to the enhanced solution concentration values observed during dissolution of the amorphous solid dispersions.
Jansens et al. [17] studied physical chemistry of solid dispersion. They discussed the strategies that include complete removal of drug crystallinity, and molecular dispersion of the poorly soluble compound in a hydrophilic polymeric carrier. They concluded that to reduce development times and to arrive at a more rational excipient selection strategy, understanding fundamental aspects of solid dispersions is imperative. Thus the formulation compounds should be selected on the basis of their stabilizing effect on supersaturated solutions formed upon release, as well as their effect on the shelf life stability of the glass solution.
Park et al. [18] studied the Physicochemical Characterizations of Tacrolimus loaded Solid dispersion with Sodium Lauryl Sulfate and Sodium Carboxylmethyl Cellulose. They concluded that the solid dispersion at the tacrolimus/CMC-Na/sodium lauryl sulfate/citric acid ratio of 3/24/3/0.2 significantly improved the drug solubility and dissolution compared to powder. Thus, the solid dispersion system with water, sodium lauryl sulfate, citric acid and Na-CMC should be a potential candidate for conveying a poorly water-soluble tacrolimus with an elevated solubility and no convertible crystalline.
Badry et al. [19] prepared and characterized the solid dispersions of non-steroidal anti-inflammatory drug, Indomethacin (IND),which is basically water insoluble, with polyethylene glycol 4000 (PEG4000) and Gelucire 50/13 (Gelu.) for enhancing the dissolution rate of the drug. An enhanced dissolution rate of IND at pH 7.4 and 1.2 was observed when the drug was dispersed in these carriers in the form of physical mixtures (PMs) or SDs. IND released faster from the SDs than that from the pure crystalline drug or the PMs. The dissolution rate of IND from its PMs or SDs got increased with an increasing amount of polymer.
Shah et al. [20] developed solid dispersions of valdecoxib with an objective of dissolution enhancement by melt granulation technique by using polyvinyl pyrollidone (PVP K 30) and polyethylene glycol (PEG 4000) in both alone (1:1) and in combination (1:0.5:0.5). In vitro dissolution studies performed in 0.1 N HCl has shown a significant enhancement in dissolution rate while PVP K 30 and PEG 4000 were used in combination.Improved drug dissolution by both the carriers may be attributed to the reduction in drug crystallinity, improved wettability and solubilizing effects from solid dispersions of valdecoxib.
Almeida et al. [21] investigated the formation of finasteride: PEG 6000 and finasteride:Kollidon K25 solid dispersions and finasteride:b-cyclodextrin inclusion complexes by solvent evaporation method using a mixture of water:ethanol (1:1). They concluded that dissolution rate of solid dispersions and inclusion complexes was significantly greater than that of pure drug as well as its corresponding physical mixtures, thus indicating the formation of solid dispersions and inclusion complexes with an increased solubility of the poorly soluble drug, finasteride.
Potluri et al. [22] studied the enhancement of dissolution of poorly soluble carvedilol by solid dispersions (SDs) with Gelucire 50/13 using solvent evaporation method. From the dissolution parameters such as dissolution efficiency, mean dissolution time and drug release rate, an improved dissolution characteristics for SDs were observed in comparison with physical mixture and pure drug. Thus they concluded that SDs of carvedilol in Gelucire 50/13 showed enhanced solubility and dissolution rate compared to pure drug.
Kushwaha et al. [23] studied to improve the dissolution rate of Acyclovir, a poorly water soluble drug by solid dispersion technique using a water soluble carrier, PEG-6000, urea, mannitol. The solid dispersions were developed by co-grinding method, physical method and solvent evaporation method. The prepared solid dispersions possess an increment in dissolution rate and solubility compared to the plain drug.
Akiladevi et al. [24] prepared solid dispersion of Paracetamol by physical triturating method, and fusion method by using 1:1, 1:4 and 1:5 ratios of drug and polymers (PEG 4000, PEG 6000 and urea). Hence it was concluded that the dissolution of Paracetamol could be improved by the solid dispersion and the PEG6000 based solid dispersions were more effective in enhancing the dissolution rate in comparison to others.
Lim et al. [25] studied an emulsified solid dispersion of Docetaxel. Various emulsifying pharmaceutical excipients and a super saturation promoter like hydroxypropyl methylcellulose (HPMC) were introduced into the PEG6000- based solid dispersion to further improve its solubilizing capability. Kim et al. [26] investigated the solid dispersion formulations of Mosapride with controlled release characteristic using various polymers, elucidate the release mechanism, and characterize the interaction patterns between Mosapride and polymers. The results indicated that the solid dispersion formulation containing PVP/Eudragit RSPO or HPMC mixture could serve as a good controlled- release system for Mosapride.
References
- Krishnamoorthy V, Nagalingam A, Priya Ranjan Prasad V, Parameshwaran S, George N, et al. (2011) Characterization of Olanzapine-Solid Dispersions. Iran J Pharm Res 10(1): 13-24.?
- Leuner C, Dressman J (2000) Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm 50(1): 47-60.
- Aulton ME (1998) Pharmaceutics: The science of dosage form design (10th edn). Edinburgh: Churchill Livingstone, pp 63-64.
- Costa P, Sarmento B, Vasconcelos T (2009) Solid dispersions as strategy to improve oral bioavailability of poorly water soluble drugs. Drug Discov Today 12(23-24): 1068-1075.
- Arunachalam M, Karthikeyan M (2010) Solid dispersions: A review. Current Pharm Res 1(1): 82-90.
- Mooter GV (2012) The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov Today 9(2): 9-85.
- Karavas E, Georgarakis E, Sigalas MP, Avgoustakis K, Bikiaris D (2007) Investigation of the release mechanism of a sparingly water-soluble drug from solid dispersions in hydrophilic carriers based on physical state of drug, particle size distribution and drug-polymer interactions. Eur J Pharm Biopharm 66(3): 334-347.
- Kapoor B, Kaur R, Kaur S (2012) Solid Dispersion: An Evolutionary Approach for Solubility Enhancement of Poorly Water Soluble Drugs. Int J Rec Adv Pharm Res 2(2): 1-16.
- Giri KT, Kumar K (2012) Bulletin of Faculty of Pharmacy, Cairo University 7(2): 1-7.
- Mura P, Fauccia MT (2001) The influence of polyvinylpyrrolidone on naproxen complexation with hydroxypropyl-b-cyclodextrin. Eur J Pharm Sci 13(2): 187-194.
- Ruan LP, Yu BY, Fu GM, Zhu DN (2005) Improving the solubility of ampelopsin by solid dispersions and inclusion complexes. J Pharm Biomed Anal 38(3): 457-464.
- Chauhan B, Shimpi S, Paradkar A (2005) Preparation and evaluation of glibenclamide-polyglycolized glycerides solid dispersions with silicon dioxide by spray drying technique. Eur J Pharm Sci 26: 219-30.
- Heo MY, Piao ZZ, Kim TW, Cao QR, Kim A, et al. (2005) Effect of solubilizing and microemulsifying excipients in polyethylene glycol 6000 solid dispersion on enhanced dissolution and bioavailability of ketoconazole. Arch Pharm Res 28(5): 604-611.
- Urbanetz NA, Lippold BC (2005) Solid dispersions of nimodipine and polyethylene glycol 2000: dissolution properties and physico-chemical characterisation. Eur J Pharm Biopharm. 59(1): 107-118.
- Ansari MT, Sunderland VB. Solid Dispersions of Dihydroartemisinin in Polyvinylpyrrolidone. Arch Pharm Res 31(3): 390-398.
- Konno H, Handa T, Alonzo DE, Taylor LS (2008) Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur J Pharm Biopharm 70(2):493-499.
- Janssens S, Van den Mooter G (2009) Review: physical chemistry of solid dispersions. J Pharm Pharmacol 61(12): 1571-1586.
- Park YJ, Ryu DS, Li DX, Quan QZ, Oh DH, et al. (2009) Physicochemical characterization of tacrolimus-loaded solid dispersion with sodium carboxylmethyl cellulose and sodium lauryl sulfate. Arch Pharm Res 32(6): 893-898.
- Badry MEl, Fetih G, Fathy M (2009) Improvement of solubility and dissolution rate of indomethacin by solid dispersions in Gelucire 50/13 and PEG4000. Saudi Pharm J 17(3): 217-225.
- Saha J, Vasanti S, Nair A, Vyas H (2009) Enhancement of dissolution rate of valdecoxib by solid dispersions technique with PVP K 30 & PEG 4000: Preparation and in vitro evaluation. J Inclus Phenom 63(1): 6975.
- Almeida HM, Marques C (2011) Physicochemical characterization of finasteride: PEG 6000 and finasteride: Kollidon K25 solid dispersions, and finasteride: β-cyclodextrininclusion complexes. Journal of Inclusion Phenomena and Macrocyclic Chemistry 70(3-4): 397-406.
- Potluri HK, Bandari S, Jukanti R, Reddy P, Reddy V (2011) Solubility Enhancement and Physicochemical Characterization of Carvedilol Solid Dispersion With Gelucire 50/13. Arch Pharm Res 34(1): 51-57.
- Kushwaha A, Prajapati SK, Sharma B (2011) Comparative Study of Acyclovir Solid Dispersion for Bioavailability Enhancement. Amer J Pharm Tech Res 1(3): 179-201.
- Akiladevi D, Shanmugapandiyan P, Jebasingh D, Basak S (2011) Preparation and evaluation of Paracetamol solid dispersion technique. Int J Phar Pharm Sci 3(1): 188-191.
- Lim SM, Pang ZW, Tan HY, Shaikh M, Adinarayana G. et al. (2015) Enhancement of docetaxel solubility using binary and ternary solid dispersion systems. Drug Dev Ind Pharm 41(11): 1847-1855.
- Kim HJ, Lee SH, Lim EA (2011) Formulation optimization of solid dispersion of Mosapride hydrochloride. Arch Pharm Res 34(9): 1467.






























