The S-Methyl-(2-Methoxycarbonylamino-Benzimidazole-5) Thiosulfonate as Potential Anticancer Agents
Lubenets VI * ,Parashchyn ZhD, Vasylyuk SV, Novikov VP
Department of Technology of Biologically Active Substances, Pharmacy and Biotechnology, Ukraine
Submission: June 05, 2017; Published: July 03, 2017
*Corresponding author: Lubenets VI, Department of Technology of Biologically Active Substances, Pharmacy and Biotechnology, Ukraine, Tel: +380973323012; Email: vlubenets@gmail.com
How to cite this article: Lubenets VI, Parashchyn ZhD, Vasylyuk SV, Novikov VP. The S-Methyl-(2-Methoxycarbonylamino-Benzimidazole-5) Thiosulfonate as Potential Anticancer Agents. Glob J Pharmaceu Sci. 2017; 3(2): 555607. DOI: 10.19080/GJPPS.2017.03.555607
Abstract
In-vitro anticancer activity of the S-methyl-(2-methoxycarbonylamino-benzimidazole-5) thiosulfonate (NSC NCI code - 713598 - J/1) was tested by the National Cancer Institute and it has revealed the anticancer activity on leukemia, melanoma, lung, colon, CNS, ovarian, renal, prostate and breast cancers cell lines.
Keywords: Thiosulphonates; Anti-cancer activity
Introduction
It is known that sulfur-containing organic compounds, including thiosulfonic acids and their derivatives are potential biologically active compounds with broad spectrum of action based on their ability to participate in nitrogen metabolism, in the synthesis of enzymes, hormones, proteins necessary for normal functioning of body [1]. Given the high synthetic and pharmacological potential of the derivatives of thiosulfonic acids very important is the designing of systems that would contain various combinations of thiosulfonate moiety with heterocyclic systems as this is likely to lead to the emergence of a new or modifications of the existing biological activity.
Taking into consideration the mentioned above, S-methyl- (2-methoxycarbonylamino-benzimidazole-5) thiosulfonate was the object of our researches.
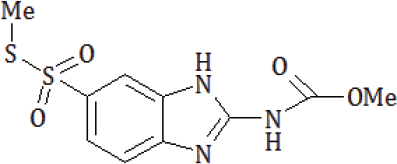
Methods of obtaining the compound and its characteristics are presented in the previous work [2]
Results and Discussion
The S-methyl-(2-methoxycarbonylamino-benzimidazole-5) thiosulfonate (NSC NCI code - 713598 - J/1) was selected by the National Cancer Institute (NCI) within the Developmental Therapeutic Program (www.dtp.nci.nih.gov) for in-vitro cell line screening. Anticancer assays were performed according to US NCI protocol, which was described elsewhere [3-6]. Primary anticancer assays of compound at one dose (concentration 10-4M) were performed in the 3-cell line panel consisting of NCI-H460 (Lung), MCF7 (Breast) and SF-268 (CNS) cancer cell lines.
The tested compound showed impressive levels of anticancer activity and it was tested in a five concentrations assay on 60 tumor cell lines over a range of concentrations (10-4 - 10-8). The cytotoxic and/or growth inhibitory effects of the reported compound were tested in vitro against the human tumor cell lines derived from nine neoplastic diseases such as leukemia, melanoma, non-small cell lung cancer, breast cancer, colon cancer, ovarian cancer, renal cancer, prostate cancer (Table 1).
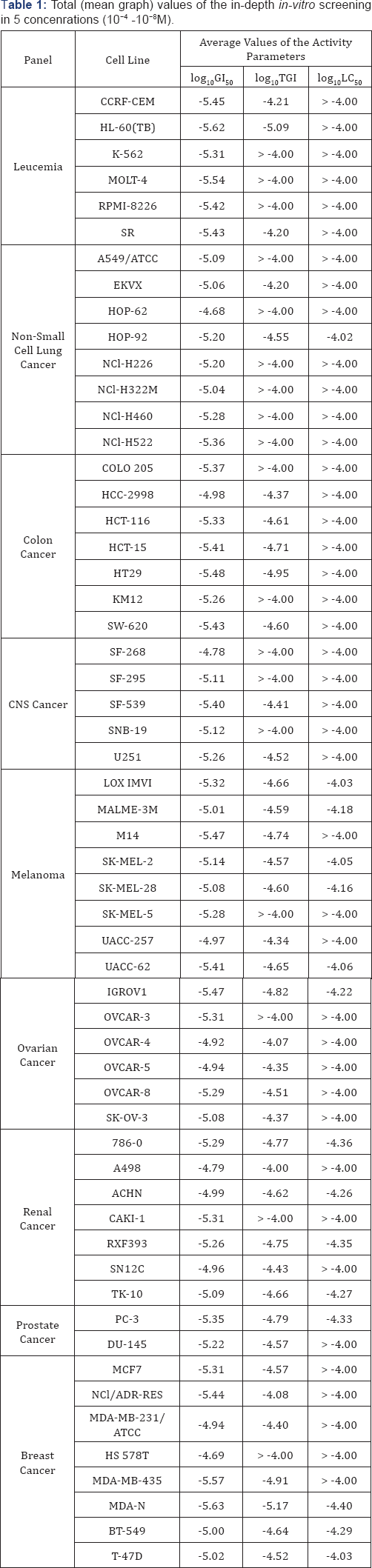
In this assay three dose-response parameters are obtained:
o growth inhibition of 50% - GI50;
o total growth inhibition - TGI;
o LC50.
Whereas the GI50 may be viewed as a growth-inhibitory level of effect, the TGI signifies a "total growth inhibition" or cytostatic level of effect. The LC50 is the lethal concentration, "net cell killing” or cytotoxity parameter. If tested parameters (log10GI50, log10TGI and log10LC50) specified in negative log units have values -4.00, then these compounds are assigned as active [7,8]. Analyzing the results of a detailed in-vitro screening tested S-methyl-(2-methoxycarbonylamino-benzimidazole-5) thiosulfonate (NSC NCI code - 713598 - J/1) showed significant growth inhibition; the average value of log10GI50 ranges from -4.00 to -5.63.
Acknowledgement
We thank National Cancer Institute, Bethesda, MD, USA, for in-vitro evaluation of anticancer activity.
References
- Lubenets V, Vasylyuk S, Monka N, Bolibrukh K, Komarovska PO, et al. (2017) Synthesis and antimicrobial properties of 4-acylaminobenzenethiosulfoacid S-esters. Saudi Pharm J 25(2): 266274.
- Parashchyn ZhD, Lubenets VI, Novikov VP (1998) Thiosulphonates- derivatives of benzimidazole. Russian Journal of Organic Chemistry 34(2): 280-284.
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, et al. (1991) Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. J Nat Cancer Inst 83(11): 757766.
- Boyd MR, Paull KD (1995) Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Dev Res 34(2): 91-109.
- Boyd MR (1997) In: Cancer Drug Discovery and Development. Teicher BA(Ed) Volume 2. Totowa, NJ, Humana Press pp. 23-43, USA.
- Shoemaker RH (2006) The NCI60 human tumour cell line anticancer drug Screen. Nat Rev Cancer 6(10): 813-823.
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, et al. (1988) Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 48(3): 589601.
- Grever MR, Schepartz SA, Chabner BA (1992) The National Cancer Institute: cancer drug discovery and development program. Semin Oncol 19(6): 622-638.






























