Simultaneous UV Spectrophotometric Estimation of Acebutalol Hydrochloride and Hydrochlorothiazide in Bulk and Combined Tablet Dosage Form
Pawar Seemarani*, Jadhav S, Tamboli A, Shaikh A and Mali S
Department of Pharmaceutical chemistry, Sahyadri College of Pharmacy, India
Submission: March 06, 2017;Published: June 30, 2017
*Corresponding author: Pawar Seemarani, Department of Pharmaceutical chemistry, Sahyadri College of Pharmacy, India, Tel: 9594780065; Email: Seema.pawar89@yahoo.com
How to cite this article: Pawar S, Jadhav S, Tamboli A, Shaikh A, Mali S. Simultaneous UV Spectrophotometric Estimation of Acebutalol Hydrochloride and Hydrochlorothiazide in Bulk and Combined Tablet Dosage Form. Glob J Pharmaceu Sci. 2017; 3(1) : 555604. DOI: 10.19080/GJPPS.2017.03.555604
Abstract
There is not a single analytical methods appeared in the literature for combined estimation of Acebutalol Hydrochloride and Hydrochlorothiazide in tablets dosage form. Attempts were made to develop a simple, precise and accurate Simultaneous UV spectroscopic method of Acebutalol Hydrochloride and Hydrochlorothiazide in bulk and Sectrazide tablet dosage form by using simultaneous equation method. UV spectrophotometric method was developed and validated as per ICH guidelines using methanol as mobile phase. Acebutalol Hydrochloride and Hydrochlorothiazide individually follows the Beer-Lamberts law over concentration range 3-18μg/ml and 1-6μg/ml, regression of coefficient was found to be r2=0.9999 and r2=0.9999 respectively. The percentage recovery was found in the range of 98% to 102% at three different levels. The proposed method was successfully applied for the determination of Acebutalol Hydrochloride and Hydrochlorothiazide in tablets dosage form as per ICH guidelines the result of the analysis were validated statistically and were found to be satisfactory.
Keywords: Acebutolol hydrochloride; Hydrochlorothiazide; Simultaneous equation; Validation; UV Spectrophotometer
Introduction
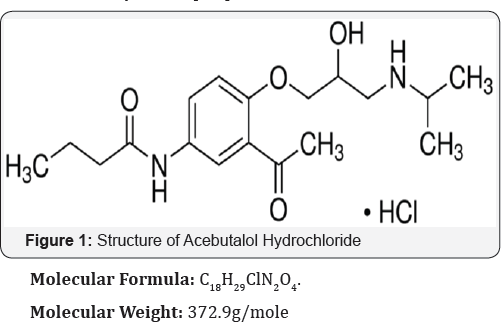
Acebutolol hydrochloride
Chemically (N-[3-Acetyl-4-[2-hydroxy-3[(1-methylethyl) amino] propoxy] phenyl] butanamide) Acebutolol hydrochloride (Figure 1) isa cardioselective, hydrophilic ß-adrenoreceptor blocking agent with mild intrinsic sympathomimetric activity (ISA) for use in treating patients with hypertension and ventricular arrhythmias[1-3]
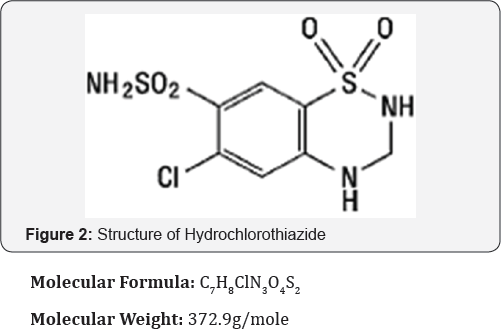
Hydrochlorothiazide
Chemically (6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazme- 7-sulphonamide1,1-dioxide) Hydrochlorothiazide is a thiazide class of diuretics used to reduces blood volume by acting on the kidneys to reduce sodium (Na) reabsorption in the distal convoluted tubule [4] (Figure 2).
Objective
The objective of the present study was to develop new analytical UV spectrophotometry method and its validation parameters for the proposed method according to ICH guidelines for the estimation of Acebutolol hydrochloride and Hydrochlorothiazide in tablets dosage form. Attempts were made to develop a simple, precise and accurate Simultaneous UV spectroscopic method.
Materials and methods
Chemical and reagents
Acebutolol hydrochloride and Hydrochlorothiazide [bulk drug] used were of analytical reagent grade purchased from Marksons Pharmaceutical Industry, Pvt. Ltd. Verana, Goa, India, methanol (AR grade) were purchased from Research lab fine chem. Industries Mumbai and double distilled water was used throughout the analysis.
Instrumentation
A shimadzu 1800UV/VIS double beam spectrophotometer with 1cm matched quartz cells was used for all spectral measurements [5].
Preparation of standard stock solution
10mg of Acebutalol and 10mg of Hydrochlorothiazide were weighed accurately and transferred to a separate 10ml volumetric flask, dissolved in sufficient quantity of methanol then sonicated for 15min and diluted to 10 ml with the same solvent so as to get the concentration of 1000μg/ml [6].
Determination of absorption maxima
Appropriate dilution of two drugs were prepared separately using standard stock solutions containing Acebutalol Hydrochloride and Hydrochlorothiazide were scanned in the range of 400nm to 200nm to determine the wavelength of maximum absorption for both the drugs. Acebutalol Hydrochloride and Hydrochlorothiazide showed absorbance maxima at 234nm and 224nm respectively. The overlain spectra showed max of both drugs (Figure 3) [7-12]
max of both drugs (Figure 3) [7-12]
Analysis of standard mixture by proposed method
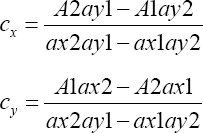
Where,
Cx = concentration of Acebutalol Hydrochloride
Cy = concentration of Hydrochlorothiazide
ax1 = absorptivity value of Acebutalol Hydrochloride at 234nm.
ax2 = absorptivity value of Acebutalol Hydrochloride at 224nm.
ay1= absorptivity value of Hydrochlorothiazideat 234nm.
ay2= absorptivity value of Hydrochlorothiazide at 224nm.
A1 = absorbance of standard mixture at 234nm.
A2 = absorbance of standard mixture at 224nm.
Analysis of marketed formulation by proposed method
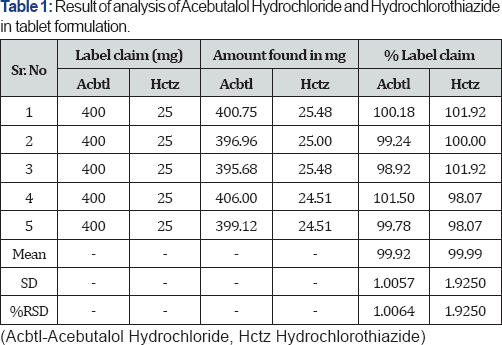
Ten tablets of brand name Sectrazide were used. A quantity of tablet powder equivalent to Acebutalol Hydrochloride (10mg) and Hydrochlorothiazide (10mg) was transferred to 10ml volumetric flask and dissolved in methanol. The aliquot portion of filtrate was further diluted to get Acebutalol Hydrochloride (160ug/ml) and Hydrochlorothiazide (10ug/ml) respectively (Table 1).
Method validation
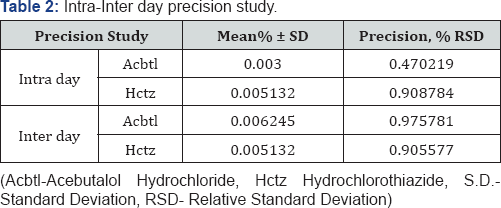
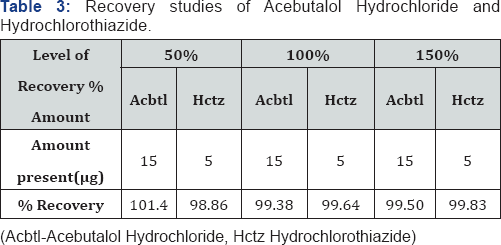
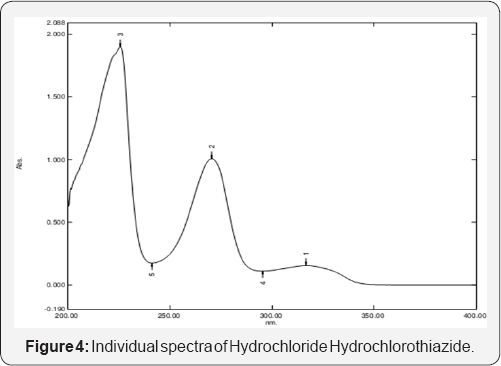
The method is developed and validated according to analytical procedure as per the ICH guidelines for validation of analytical procedures. All the parameters such as linearity, precision, LOD, LOQ and accuracy for the analytes were found to be within the limit and satisfactory. The recovery studies showed that the result were within the limit indicating no interference (Table 2 & 3) (Figure 4) [13,14].
Results and Discussion
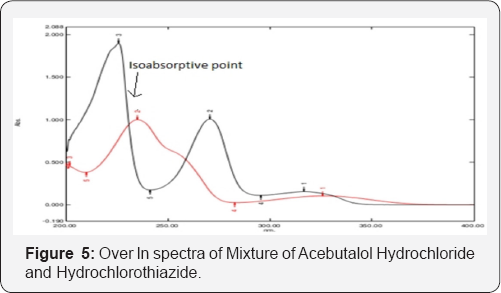
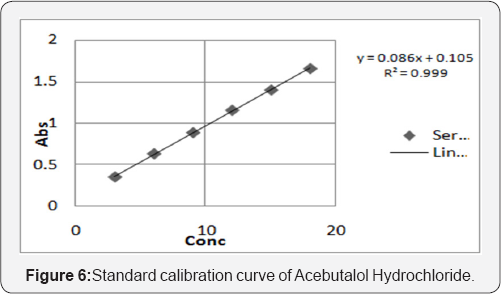
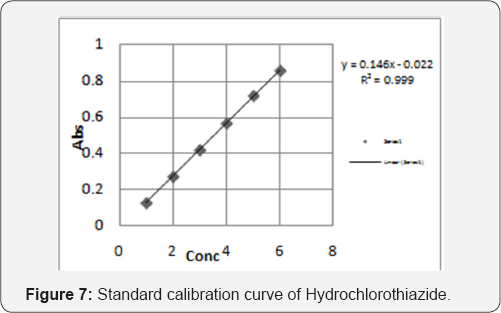
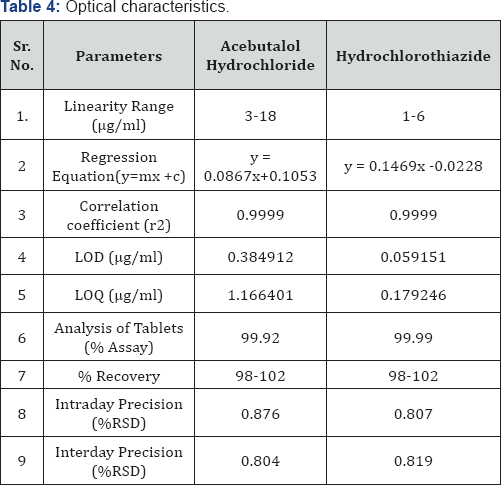
From the individual spectra of Acebutalol Hydrochloride and Hydrochlorthiazide in methanol (Figure 5 & 6) at concentration of 10ug/ml of Acebutalol Hydrochloride and 10μg/ml Hydrochlorthiazide, two wavelengths 234nm and 224nm were selected for simultaneous estimation of drugs respectively.The relation between concentration and absorbance for individual drug was studied. Acebutalol Hydrochloride and Hydrochlorothiazide individually follows the Beer-Lamberts law over concentration range 3-18μg/ml and 1-6μg/ml respectively. The absorptivity values for both the drugs were determined at the selected wavelengths for Acebutalol Hydrochloride and Hydrochlorthiazide respectively. Validation result is shown in the Table 4 [15,16] (Figure 7).

Conclusion
The proposed method is simple, accurate, precise and selective for the estimation of Acebutalol Hydrochloride and Hydrochlorothiazide. The method is economical, rapid and do not require any sophisticated instruments contrast to chromatographic method. The method was found to provide high degree of precision and reproducibility. It can be effectively applied for the routine analysis of Acebutolol hydrochloride and hydrochlorothiazide in bulk drug and in combine tablet dosage form.
References
- Zaveri M, Amit K (2010) Development and Validation of A RP-HPLC For The Simultaneous Estimation Of Atenolol And Hydrochlorothiazide In Pharmaceutical Dosage Forms. International Journal of Advances in Pharmaceutical Sciences 1(2): 167-171.
- www.drugbank.com
- www.drugs.com
- Eswarudua MM, Junapudia S, Narendra T (2011) RP-HPLC method Development and Validation for Simultaneous estimation of Montelukast sodium and Levocetirizine dihydrochloride in tablet dosage form. International Journal of Pharma world Research 2(4): 1-18.
- Somkuwar S, Pathak AK (2012) Simultaneous estimation of Levocetirizine dihydrochloride and Montelukast sodium by RP-HPLC method. Journal of Pharmacia 1(3): 91-94.
- Patel NK, Patel S, Pancholi SS (2012) HPLC method development and validation for simultaneous estimation of Montelukast sodium and Levocetrizine dihydrochloride in pharmaceutical dosage forms.International Journal of Pharmacy and Pharmaceutical Sciences 4(2): 241-243.
- Choudhari V, Kale A, Abnawe S, Kuchekar B, Gawli V, et al. (2010) Simultaneous determination of Montelukast sodium and Levocetirizine dihydrochloride in pharmaceutical preparations by Ratio Derivative Spectroscopy. International Journal of pharm tech research 2(1): 0409.
- Pallavi K, Babu SP (2012) Validated UV-Spectroscopic method for estimation of Montelukast sodium from bulk and tablet formulation. International Journal of Advances in Pharmacy 1(4): 434-437.
- Skoog DA, West DM, Holler FJ, Crouch SR (2007) Fundamental of analytical chemistry (8th edn), Thomson Brooks/Cole pp.1-5.
- Willard HH, Merritt LL, Dean JA, Frank AS (1986) Instrumental method of analysis (7th edn) CBS publishers and Distributors, New Delhi, India, pp.1-5.
- Connors KA (1999) Text Book of Pharmaceutical Analysis (3rd edn) Jhon willey & sons pp. 341.
- Chatwal GR, Anand SK (2002) Instrumental Method of Chemical Analysis (5th edn), pp. 2.626-2.628.
- Kasture AV, Mahadik KR, Wadodkar SG, More HN (2002) Text book of Pharmaceuticals Analysis Instrumental Methods pp. 48-50.
- Bekett AH, Stenlake JB (2002) Practical Pharmaceutical Chemistry, CBS Publishers and Distributors, New Delhi, India, Part-2 pp. 275-337.
- ICH Q2A (1994) Text on validation of analytical procedures, International conference on harmonization, Tripartite guideline pp. 1-5.
- ICH Q2B Validation of analytical procedures: methodology, International conference on harmonization, Tripartite guideline pp. 1-10.






























