A Review of Dalvance for the Treatment of Acute Bacterial Skin and Skin Structure Infections (Absssis)
Sanyaolu A1* Abioye A2, Alaofin B3, Johnson G3, and Alhussaini A3
1Nigeria Center for Disease Control, Federal Ministry of Health, Nigeria
2Department of Pharmacy, Leicester School of Pharmacy, United Kingdom
3Saint James School of Medicine Anguilla, BWI
Submission: May 30, 2017; Published: June 06, 2017
*Corresponding author: Adekunle Sanyaolu, Nigeria Center for Disease Control, Federal Ministry of Health, Nigeria; Email: sanyakunle@gmail.com
How to cite this article: Sanyaolu A, Abioye A, Alaofin B, Johnson G, Alhussaini A. A Review of Dalvance for the Treatment of Acute Bacterial Skin and Skin 007 Structure Infections (Absssis). Glob J Pharmaceu Sci. (2017) 2(4): 555595. DOI: 10.19080/GJPPS.2017.02.555595
Abstract
Gram positive Bacteria can cause resistant to the antibiotics which are currently available for treating acute bacterial skin and skin structure infections (ABSSSIs) thereby rendering the antibiotics unsafe to use for treatment. The production of new drugs for the treatment of this infection as well as other infections caused by the resistant gram positive organisms is imperative for public health. MRSA is a resistant pathogen of special importance in the clinical setting as it is difficult to treat and easy to spread both in the healthcare settings as well as in the community. The newest antibiotic, Dalvance, is promising. It is as effective as other drug protocols like the combination of vancomycin and linezolid. So far, no resistance has been produced against the drug with over ten years in clinical trials. This review discusses the drug description, indications and usage, side effects, drug interactions and non-clinical toxicology, clinical pharmacology, mechanism of action, pharmacodynamics, pharmacokinetics, microbial efficacy, studies, dosage and administration information, of the new drug Dalvance and its importance in the health care system for the treatment of ABSSSIs.
Keywords: Dalvance; Antibiotics; MRSA; ABSSSI
Abbreviations: MRSA: Methicillin Resistance Staphylococcus aureus; ABSSSI: Acute Bacterial Skin and Skin Structure Infection; MSSA: Methicillin Susceptible Staphylococcus aureus; MRSE: Methicillin-Resistant Staphylococcus epidermidis; CDAD: Clostridium difficile-Associated Diarrhea; Cmax: Maximum Concentration; AUCO: Area Under the Curve Origin; CYP450: Cytochrome p450; CRCL: Creatinine Clearance; CE: Clinically Evaluable
Introduction
There is the need to develop new antibiotic due to the emergence and spread of Gram- positive bacteria that are resistant to nearly all commercially available antibiotics that are currently in use for the treatment of acute bacterial skin and skin structure infection (ABSSSI) caused by microorganisms such as Methicillin Resistance S. aureus (MRSA) and Methicillin susceptible S. aureus (MSSA) infections. The infection generally present with cellulitis, draining pus, and abscess, which are the first signs that MRSA has taken hold of a wound. Final diagnosis must be done via culture, and antibiotic sensitivity testing of the bacteria found at the wound site. A PCR test is also available to pinpoint the exact kind of bacteria that is causing infection [1,2].
MRSA has become a very dangerous pathogen because it has mutated to grow resistance to multiple antibiotics. In order to determine the best antibiotic therapy, all MRSA strains would have to be isolated. Hospital or clinic acquired MRSA infections are often treated with vancomycin or a combination of other antibiotics given intravenously. Community-acquired MRSA can be treated with oral or topic antibiotics on an outpatient basis. If the community acquired MRSA is severe enough, as in the cases of pneumonia, intravenous treatment might be necessary [3].
In order to prevent the spread of MRSA, excellent hygiene is mandatory, especially by those in health care facilities. Avoiding contact with those who are known to be infected, wearing disposable gloves, gowns, and other person protective equipment when treating MRSA patients is imperative. Keeping abrasions and minor lacerations in the skin covered will help to prevent the spread of the bacteria, especially in children and athletes.
MRSA is just one of the 18 pathogens which the CDC has listed as a multidrug-resistant microbe, otherwise known as a “superbug.” As more of these pathogens are added to the list,it is imperative that new antibacterial agents are created. It is necessary for public health to be able to fight infections that are difficult to treat. Dalvance is the newest drug on the market which shows promise as having comparable efficacy as older drugs and over the past ten years resistance has not been seen to develop [4].
Drug description
Dalvance (dalbavancin) is a new second-generation lipoglycopeptide antibiotic [5]. It is within the same class of drugs as vancomycin which is currently the most widely used treatment for methicillin-resistant S. aureus (MRSA) infection. Vancomycin is also one of the only treatments available for these people. As a semisynthetic lipoglycopeptide, Dalvance was developed to improve the natural glycopeptides which are currently available on the market [6].
Dalvance has been found to have in vitro activity against Gram-positive pathogens. Some of these pathogens include MRSA and methicillin-resistant S. epidermidis (MRSE), which are some of the most difficult infections to treat [7]. The Proper dosage of the drug is through two doses, one week apart [8]. Phase 3 clinical trials have been completed in adults with complicated skin infections for Dalvance but in December 2007 the FDA decided that more data was necessary before approval could take place [9]. Pfizer announced on September 9, 2008, that it would suspend all marketing programs in order to produce another Phase 3 study of Dalvance. In December 2009, Durata Therapeutics acquired the rights to the drug and began two new Phase 3 studies with patients with ABSSSI.
Preliminary results which were attained in 2012 showed promise. A total of 1,289 adults with ABSSSI were included in studies comparing Dalvance with vancomycin. It was found to be about as effective as this previous drug and with resistance being such a problem, a new additional medication was necessary on the market. In May 2014, The Food and Drug Administration approved Dalvance for the treatment of ABSSSIs. It is specifically approved for susceptible bacteria such as S. aureus, and both Methicillin-susceptible and methicillin-resistant strains of S.pyogenes [10].
Dalvance (dalbavancin) is an injection made of lipoglycopeptide which is synthesized from a fermented product of the Nonomuraea species. It is a mixture of homologs which are closely related with the largest component. These homologues have the same core structure and only differ regarding the side chain of fatty acids of the N-acylamino glucuronic acid moiety (R1) as well as the presence of another methyl group (R2) at the last amino group. In the most common homologs, R1 is CH2CH(CH3)2, R2 is H, which makes Dalvance's molecular formula is C88H100Cl2N10O28. The molecular weight is 1816.7 [11].
Dalvance is available in clear glass vials. It is a sterile, preservative-free, lyophilized solid powder. It appears white, off-white, or pale yellow. Each vial contains dalbavancin HCl in the equivalent of 500mg of anhydrous dalbavancin as the active ingredient. It also includes 129mg of lactose monohydrate and 129mg mannitol as excipients. At the time of manufacture, the pH of the product may need to be adjusted therefore sodium hydroxide or hydrochloric acid may be added to the solution. The powder in the vial needs to be reconstituted and diluted prior to intravenous infusions in order to work properly in the human body [12].
Indications & usage
Dalvance is indicated for use for treating acute bacterial infections of the skin and skin structures, specifically in adult patients. The acute bacterial skin and skin structure infections (ABSSSI) that are susceptible to Dalvance includes isolates of Gram-positive microorganisms such as S. aureus (both Methicillin-susceptible and methicillin-resistant strains), S. agalactia, S. pyrogens, and the S. anginosus group which includes S. intermedius, S. anginosus, and S. constellatus.
All efforts should be made to prevent the maturation of drug- resistant bacteria in order to maintain the effectiveness of all antibacterial agents, and especially Dalvance [13]. It should only be used to treat infections which are known to be susceptible to the drug, or those which are highly suspected to be susceptible. In circumstances when culture and sensitivity tests are available, they should be consulted prior to using Dalvance in a treatment plan. When these tests are not available when choosing or modify an antibacterial therapy, local epidemiology, and susceptibility patterns can help in the selection of therapy.
Resistance to antibiotics occurs when the pathogen is able to change in such a way as the efficacy of the medications to treat, cure or prevent the infection is eliminated. The bacteria are able to survive the conditions and multiply in order to cause even more harm. Some bacteria are able to neutralize the antibiotic; others can remove the antibiotic, and others can change how the antibacterial attempts to attack the bacteria [14].
If Dalvance is not used on appropriate bacteria or is not administered as indicated, there is the potential for bacteria to become resistant to this drug as well. Antibiotics work by killing or inhibiting the growth of only the particular bacteria that it works against. Occasionally, some of the bacteria survive because it has the ability to escape or neutralize the effect of the antibiotic. Those few (or even just one) bacterium multiplies and replaces the other bacteria which did succumb to the antibiotic. Following courses of the same antibiotic will produce the same results by not killing the original or offspring bacteria. Bacteria can also become resistant to a particular antibiotic through genetic mutation, making it a different strain which no longer is affected by the medication. Because the DNA for resistance can be classified into different bacteria that can becomes resistant to multiple antibiotics at the same time. Hence, maintaining strong usage and indication protocol is imperative to prevent resistance.
Adverse reactions
Anaphylactic hypersensitivity reactions have been reported in patients who received Dalvance infusions. Skin reactions have also been reported. If these reactions occur, discontinue use of Dalvance. Prior to use, the patient should be screened for any previous hypersensitivity to glycopeptides. As there may be cross-sensitivity, these patients should be given Dalvance with caution [15].
Dalvance is given as an intravenous infusion over 30minutes. This is done in order to reduce the risk of reactions related to the infusion. If infusion occurs too quickly, Dalvance can cause systemic reactions similar to “Red-Man Syndrome.” These symptoms include upper body flushing, pruritus, urticaria, and rash. Completely stopping, or slowing the infusion rate often reduces the reaction symptoms [16].
As with many patients who receive systemic antibacterial medications, Dalvance can cause C. difficile-associated diarrhea (CDAD). CDAD due to Dalvance can range from mild to severe. This occurs because antibacterial medications can alter the normal bacterial flora within the colon, which allows C. difficile to grow out of control. C. difficile produces two different toxins which lead to CDAD [17].
Adverse reactions were reported during Phase 2 and 3 clinical studies. Serious reactions occurred in 6.1% of patients. 3% of patients experienced such severe reactions which required the discontinuation of Dalvance. Some of the most common reactions were nausea, headache, and diarrhea. These reactions lasted an average of 4days and were all less frequent from Dalvance than from the comparative antibiotics. There are many different reactions which were experienced by the patients within the test groups, but they affected less than 2% of the test population. Blood and lymphatic reactions that were associated with Dalvance include anemia, both hemorrhagic and non-hemorrhagic, leukopenia, neutropenia, thrombocytopenia, petechiae, eosinophilia, and thrombocytosis.
Gastrointestinal reactions include gastrointestinal hemorrhage, melena, hematochezia, and abdominal pain. Hepatotoxicity, infusion site reactions, anaphylactoid reactions, hypoglycemia, dizziness, bronchospasm, urticaria, were also seen. C. difficile colitis, oral candidiasis, and vulvovaginal mycotic infections were found. Hepatic transaminases increased, blood alkaline phosphatase increased, and the international normalized ratio increased. Vascular reactions such as flushing, phlebitis, wound hemorrhage, and spontaneous hematoma were also noted.
No interactions have been noted between Dalvance and laboratory tests. There have yet been no clinical studies confirming any interactions between Dalvance and other drugs, but there is potential for interactions between cytochrome P450 (CYP450) substrates, inducers, or inhibitors and Dalvance. Studies have shown that the administration of inducers, inhibitors, or substrates of CYP450 does not affect the pharmacokinetics of Dalvance [18]. Individual administration of other drugs does not affect it either. Individual drugs tested include aztreonam, acetaminophen, fentanyl, furosdime, metronidazole, midazolam, simvastatin, and proton pump inhibitors such as esomeprazole, omeprazole, lansoprazole, and pantoprozaole [19].
Clinical pharmacology
Dalvance is an antibacterial medication. It's activity against bacterial agents is best correlated with the ratio of the area under the curve of concentration time for the minimal inhibitory concentration for S.aureus as seen in animal models of infection. One study was conducted in patients with complicated infections of the skin and skin structure and supported the two-dose regimen.
Pharmacokinetic parameters were discovered in healthy subjects, patients with infection, and specific populations. In healthy people, the half-life was found to be about 8.5days or 204hours. In this population, the area under the curve origin (AUCO)-24h and maximum concentration (Cmax) both increased based on the dose size after intravenous infusion. This indicates linear pharmacokinetics [20]. The mean plasma concentrationtime profile was also analyzed. It was found that in healthy people when administered as recommended, Dalvance does not accumulate in the body. This was observed over eight weeks when 1000mg of Dalvance was first administered, followed by 7weekly doses of 500mg [21].
Dalvance is bound reversibly to plasma proteins, especially albumin. It does so approximately 93% and is not affected by the drug's concentration, kidney or liver impairment. The mean concentration of the drug in skin blister fluid continues to be above 30mg/L even 7days after dosing of 1000mg. The average ratio of AUC0-144hours in skin blister fluid compared to AUC0- 144hours in plasma is between 0.44 and 0.64 [22].
Studies in-vitro with human microsomal enzymes as well as hepatocytes show that Dalvance does not inhibit, induce or act as a substrate of cytochrome p450 (CYP450) isoenzymes. A minimal metabolite of Dalvance, hydroxy-dalbavancin has been found in the urine of healthy subjects. Levels of hydroxy- dalbavancin metabolite which are quantifiable have not been found in plasma [21]. Dalvance is eliminated from the body via several different methods. After receiving one dose of 1000mg of Dalvance, healthy people excreted 20% of the dose through feces over 70days. 33% of the dose was removed from the body in urine as the unchanged chemical. An additional 12% was excreted in urine as the metabolite hydroxy-dalbavancin. All excretion through urine continued 42days post dose [22].
Patients with renal impairment were evaluated to understand how elimination varies in this population. Twenty- eight patients with differing degrees of renal impairment were compared to 15 healthy subjects. In mild renal impairment, the mean plasma clearance was reduced by 11%. Moderate renal impairment indicated a reduction of mean plasma clearance of 35% and severe cases of renal impairments caused the drug to clear out of the plasma 47% slower [23].
How the decrease of mean plasma clearance time and an associated increase AUC0-ot which were found in pharmacokinetic studies of the drug in patients with renal disease correlate has not yet been noted. Studies have found that no dosage changes need to be made in regards to patients with creatinine clearance (CRCL), which is greater than 30mL/min or in those patients which are regularly undergoing hemodialysis treatments. Patients who have diminished CRCL or who are not undergoing treatments such as hemodialysis should be given a smaller dosage of Dalvance. 750mg of Dalvance should be infused for the first dose, followed by 375mg the following week [24].
Dalvance is a semisynthetic lipoglycopeptide. It interferes with the synthesis of the cell wall by binding terminus of the D-alanyl-D-alanine terminus at the stem pentapeptide within nascent cell wall peptidoglycan. This action prevents crosslinking. It is bactericidal in the body against strains of S.aureus and S.pyogenes [25].
Microbial efficacy
Dalvance has been found to have similar results as comparative antimicrobials but with less resistance as of thus far. In clinical studies which had three different goals, the drug was found to be less efficient in the short term, but over time appears to have slightly better results. The drug was tested on specific bacteria against a comparative drug in order to understand its effectiveness compared to drugs already on the market [26]
Early responders were considered those who saw no increase or decrease in the size of the lesion and a body temperature at normal. In this case, Dalvance was successful in 80.2% of instances of S. aureus compared to 85.5% for the comparator. Success also occurred 80.2% of cases in Methicillin- susceptible cases for Dalvance and 86.2% for the comparator. Only 80.0% of methicillin-resistant strains were susceptible to Dalvance while 83.6% of the comparator cases were successful. S.agalactiae was also tested. Early responders taking Dalvance only saw success 50% of the time while 78.6% of comparator cases were successful. S. anginosus were more susceptible to Dalvance than comparator in early responders with 75.7% and 66.7% of success respectively. The S. anginosus group saw 81.8% of achievement with Dalvance and 92.0% success with the comparator [27].
The second goal tested was at least a 20% reduction in lesion size at 48 to 72hours after administration of Dalvance. At this goal, most of the bacteria tested had better success rates with Dalvance than the comparator drug. S. aureus had an overall success rate of 93.0% with Dalvance. Methicillin- susceptible species were 93.4% successful while methicillin- resistant strains were 92.2% successful. The comparative drug only saw 90.6%, 91.5%, and 88.1% success rates compactivity. S.agalactiae met the goal 83.3% with Dalvance and only 74.4% with the comparative drugs. Dalvance created 86.5% success with S.pyogenes whereas the comparative drugs were only 75.0% effective. The S.anginosus group was the only one in which the comparator drug was more effective. Dalvance was effective in 95.5% while the comparator was 100% effective.
The third and final goal was described as clinical success at day 26 to 30 post administration of the drug. In this objective, the two drugs tested had similar success rates. In this case, Dalvance was successful in 84.4% of instances of S.aureus compared to 89.5% for the comparator. Success also occurred 85.0% of cases in Methicillin-susceptible cases for Dalvance and 90.5% for the comparator. Only 83.3% of methicillin-resistant strains were susceptible to Dalvance while 85.1% of the comparator cases were successful. S.agalactiae was also tested. Early responders taking Dalvance only saw success 83.3% of the time while 78.6% of comparator cases were successful. S.pyogenes were more susceptible to Dalvance than comparator in early responders with 89.2 and 88.9% of success respectively. The S.anginosus group saw 95.5% of achievement with Dalvance and 92.0% success with the comparator.
Clinical/ case studies
In order to fully test the efficacy and safety of Dalvance, two Phase 3 studies were conducted. These studies were randomized, double-dummy, double-blind clinical trials which were similar in design to each other. The total population was 1,312 patients. One population was given Dalvance in an appropriate dosage of 1000mg followed by 500mg the following week. The other population was treated with intravenous vancomycin in the dosages of 15mg/kg every 12hours with an option to change to oral linezolid after 3days [28]. Patients with renal impairment received the appropriate dose of Dalvance. About 5% of the patients also received support for Gram-negative pathogens via a protocol-specific empiric support such as aztreonam intravenous administration [29].
About 50% of the patients in the trials were being treated for cellulitis. About 30% had major abscess while 20% had wound injections. The average lesion size at baseline was 341 cm2. Beyond having clinical signs and symptoms of local infection, each participant in the study also need to have at least one systemic sign of disease at the onset of the study. 85% of the study population had a body temperature of 38 °C or higher, 40% had a white blood cell count greater than 12,000 cells/mm3 and 23% had more than 10% or more band formations within their white blood cells. These are all systemic clinical signs of infection.
Both trials included a broad range of racial and cultural participants. 59% of the participants were of Eastern European descent while 36% were from North America. 89% of the participants were Caucasian while 58% of the participants were male. The average age was 50years, and the mean body mass index was 29.1kg/m2. The end point which was desired during these trials was a clinical response to the medication. This was defined as no increase in lesion size after 48 to 72hours after administration of therapy and a body temperature at or below 37.6 °C upon repeated measurement. 83% of the patients that received Dalvance in the first trial reached the end point goal while 76.8% did the same in the second trial. 81.8% of the vancomycin/ linezolid patients reached the end point goal in the first trial and 78.3% in the second (Figure 1).

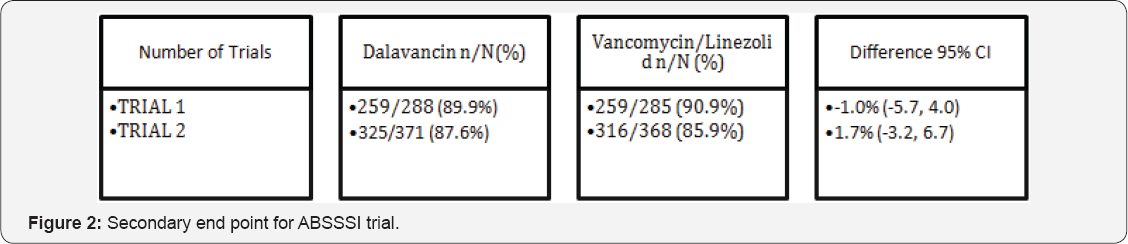
A secondary end point goal in these ABSSSI trials was a decrease in lesion size during the same time frame. During the first trial, 89.9% of Dalvance patients actually saw a reducing in the size of their lesions, similarly 87.6% of the second trial patients experienced a reduction. Vancomycin had results that were comparable to Dalvance with 90.9% reducing in the first trial and 85.9% in the second trial (Figure 2). An additional secondary endpoint which was tested was the success rate determined at follow-up visits which took place between 26 and 30days after the beginning of treatment with Dalvance.Clinical success was documented as decrease in both width and length of the lesion, a body temperature of 37.6 °C or lower, and lacking local signs such as purulent discharge and drainage improved from baseline, no heat/warmth, swelling/induration or tenderness upon palpation. In order to test this secondary endpoint, two different groups were tested in each trial and medicine tested. These are the Intent-to-Treat (ITT) groups which have affiliated infections which needed treatment, and the clinically evaluable (CE) groups.
The ITT group met the goal 83.7% and 88.1% when under the care of Dalvance. The same group, given vancomycin/Linezolid instead fared similarly, reaching the goal 88.1% and 94.5%. The CE groups had even better results. In the first and second trials for the CE groups taking Dalvance, the clinical success rates were 93.8% and 96.3% respectively. The Vancomycin/Linezolid CE groups fared just as well. The first trial group had 96.1% success and the second group had 94.5% [30].
Dosage & administration
Dalvance is available in single-use, clear glass vials containing sterile white, off white, to pale yellow powder. It is equivalent to 500mg of anhydrous Dalbavancin. For the treatment of adults with ABSSSI, two doses of Dalvance are recommended. The first should be 1000mg, with a second dose of 500mg administered one week later [31]. The entire dosage should be administered via intravenous infusion over a time of 30minutes [32].
Saline-based solutions used for infusions may cause Dalvance to precipitate out of solution and, therefore, should not be used.How reconstituted Dalvance interacts with other intravenous additives, medications, and substances other than 5% dextrose injection have not been tested. Therefore, co-infusion should not take place. If other medications or substances need to be given intravenously, the vein should be flushed both before and after the infusion of Dalvance.
In patients with renal impairment who are known to have creatinine elimination less than 30mL per min but do not receive hemodialysis on a regular basis, the dosage should be adjusted to 750mg of Dalvance followed by the lessened dose of 375mg the following week. A renal impaired patient who does receive hemodialysis regularly scheduled do not need an adjusted dose of Dalvance regardless of the timing of hemodialysis treatments [33].
Dalvance requires preparation prior to being administered. It must be reconstituted with 25mL of sterile water through aseptic techniques in order to create one 500mg vial. The manufacturer suggests alternating gentle swirling and vial inversion to dissolve the contents completely without creating foam. Avoid shaking. The reconstituted product should be a clear, colorless or yellowish solution. Vials of reconstituted solution can be stored in refrigeration of 2 to 8 °C (36 to 46 °F) or at room temperature 20 to 25 °C (68 to 77 °F). Dalvance should not be frozen.
Dalvance can be further diluted with 5% dextrose injection to add a final concentration of 1mg/mL to 5mg/mL. This can be completed by transferring the reconstituted Dalvance aseptically to an intravenous bottle or bag that already contains 5% dextrose injection. Any unused reconstituted solution should be discarded. The intravenous bag or bottle can be stored in the same way as the solution but should not exceed 48 hours. Prior to administration, the reconstituted and diluted Dalvance should be visually inspected for any particulate or precipitate within the solution. Dispose of any solution which does not appear correct, just as any parental drugs.
Very little information regarding over dosage of Dalvance is available. A toxicity which would limit dosage has not been observed in clinical studies. During Phase 1 studies, healthy individuals were given single doses up to 1500mg and cumulative doses over 8weeks up to 450mg. No indications of toxicity or concerns laboratory results were found. Treatment for a Dalvance overdose should include supportive measurements and observation. There is no data indicating hemodialysis as a treatment for Dalvance overdose [34].
There are a few populations that should take special precaution when being administered Dalvance; as how the human body will react is not completely understood. There have been no adequate and well-controlled studies regarding the use of Dalvance in pregnant or nursing humans. In this case, the manufacturer highly urges that the benefit and potential risk be weighed heavily before choosing to use this or any other antibiotic treatments. There is a potential risk the mother, as well as the offspring and even the efficacy of the treatment, cannot be fully predicted.
Patients with renal impairment who have a known creatinine clearance level of less than 30mL/min and are not receiving hemodialysis on a regular basis should be given a dose that is two thirds of the normal recommended dose. By administering 750mg followed one week later by 375mg, patients with renal impairment can avoid potentially toxic conditions. Patients who are able to receive adequate creatinine clearance through hemodialysis do not need to make adjustments to the quantity of Dalvance which is administered or the timing of infusions.
The third population which should exercise caution when being treated with Dalvance is those with severe hepatic impairment such as those within the Child-Pugh Class B or C. There is no data available yet to determine what is a safe and appropriate dosage to treat the patient's infection and yet preventing a hepatic toxicity.
Conclusion
Because resistance to antibiotics is a dangerous condition in today’s medical world, new antiboitics needs to be developed. Resistant strains of bacteria, specifically those of S.spp. can be extremely hazardous to the patients who become infected with them. Dalvance is a new antibiotic which has been released onto the market in order to treat these infections which older generations of antibiotics are ineffective against. Dalvance is considered a second-generation antibiotic and is a lipoglycopeptide. It is similar in structure to vancomycin which is typically used to treat MRSA infections and is often the only treatment available. Dalvance, on the other hand, is semi synthetic to improve upon the natural glycopeptides which are on the market today.
Dalvance has been tested and found to be active, in vitro, against Gram-positive pathogens including MRSA, and MRSE, which is the most difficult to treat. Through proper dosing one week apart, the drug is effective against susceptible bacteria such as S.aureus, and both Methicillin-susceptible and methicillin- resistant strains of S.pyogenes.
Phase 3 clinical trials have been completed in adults with complicated skin infections for Dalvance but in December 2007 the FDA decided that more data was necessary before approval could take place. Pfizer announced on September 9, 2008, that it would suspend all marketing programs in order to produce another Phase 3 study of Dalvance. In December 2009, Durata Therapeutics acquired the rights to the drug and began two new Phase 3 studies with patients with ABSSSI. Preliminary results which were attained in 2012 showed promise. A total of 1,289 adults with ABSSSI were included in studies comparing Dalvance with vancomycin. It was found to be about as effective as this previous drug and with resistance being such a problem, a new additional medication was necessary on the market. In May 2014, The Food and Drug Administration approved Dalvance for the treatment of ABSSSIs opening new roads to the future of antibiotics.
References
- Centers for Disease Control and Prevention (2013). Methicillin- resistant Staphylococcus aureus (MRSA) Infections. Retrieved from Centers for Disease Control and Prevention.
- Thomas AGR (2015) New Drugs: Dalvance, Entyvio, Jublia, and Zontivity.
- Anderson DJ, Sexton DJ, Kanafani ZA, Auten G, Kaye KS (2007) Severe surgical site infection in community hospitals: epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 28(9): 10471053.
- Centers for Disease Control and Prevention (2010) Biggest Threats. Retrieved from Centers for Disease Control and Prevention.
- Chen M, Xu T, Zhang G, Zhao J, Gao Z, et al. (2015) High-yield production of lipoglycopeptide antibiotic A40926 using a mutant strain Nonomuraea sp. DP-13 in optimized medium. Prep Biochem Biotechnol 46(2): 171-175.?
- Jones RN, Farrell DJ, Flamm RK, Sader HS, Dunne MW, et al. (2015) Surrogate analysis of vancomycin to predict susceptible categorization of dalbavancin. Diagn Microbiol Infect Dis 82(1): 73-77.
- Scott L (2015) Dalbavancin: A Review in Acute Bacterial Skin and Skin Structure Infections. Drugs 75(11): 1281-1291.
- Gupta AK, Foley KA, Abramovits W, Rosen T (2014) Dalbavancin (Dalvance) for the treatment of acute bacterial skin infection. Skinmed 12(6): 366-369.
- Richwine L (2007) UPDATE 1-Pfizer says US FDA wants more data on antibiotic. Reuink ters.
- Yao S (2014) FDA approves Dalvance to treat skin infections. Clin Infect Dis 59(2): i.
- Chen AY, Zervos MJ, Vazquez JA (2007) Dalbavancin: a novel antimicrobial. Int J Clin Pract 61(5): 853-863.
- Durata Therapeutics US Limited (2014) Full Prescibing Information. Il: Durata Therapeutics US Limited, Chicago, USA.
- Goldstein BP, Draghi DC, Sheehan DJ, Hogan P, Sahm DF (2007) Bactericidal activity and resistance development profiling of dalbavancin. Antimicrob Agents Chemother 51(4): 1150-1154.
- Wang Q, Mao D, Luo Y (2015) Ionic Liquid Facilitates the Conjugative Transfer of Antibiotic Resistance Genes Mediated by Plasmid RP4. Environ Sci Technol 49(14): 8731-8740.
- Seltzer E, Dorr MB, Goldstein BP, Perry M, Dowell JA, et al. (2003) Once- weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis 37(10): 1298-12303.
- Traynor K (2014) Dalbavancin Approved for Acute Skin Infections. Am J Health Syst Pharm 71(13): 1062.
- Pappas A, Hanna S (2015) Dalvance by Durata Therapeutics. Pharmacy Times.
- Buckwalter M, Dowell J (2005) Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J Clin Pharmacol 45(11): 1279-1287.
- Khadem TM, van Manen RP, Brown J (2014) How safe are recently FDA- approved antimicrobials? A review of the FDA adverse event reporting system database. Pharmacotherapy 34(12): 1324-1329.
- Bradley JS, Puttagunta S, Rubino CM, Blumer JL, Dunne M, et al. (2015) Pharmacokinetics, Safety and Tolerability of Single Dose Dalbavancin in Children 12-17 Years of Age. Pediatr Infect Dis J 34(7): 748-752.
- Dunne MW, Puttagunta S, Sprenger CR, Rubino C, Van Wart S, et al. (2015) Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother 59(4): 1849-1855.
- Nicolau DP, Sun HK, Seltzer E, Buckwalter M, Dowell JA (2007) Pharmacokinetics of dalbavancin in plasma and skin blister fluid. J Antimicrob Chemother 60(3): 681-684.
- Andes D, Craig WA (2007) In-vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob Agents Chemother 51(5): 16331642.
- Bailey J, Summers KM (2008) Dalbavancin: a new lipoglycopeptide antibiotic. Am J Health Syst Pharm 65(7): 599-610.
- McCurdy SP, Jones RN, Mendes RE, Puttagunta S, Dunne MW (2015) In-vitro Activity of Dalbavancin Against Drug Resistant Staphylococcus aureus from a Global Surveillance Program. Antimicrob Agents Chemother 59(8): 5007-5009.
- Dunne MW, Sahm D, Puttagunta S (2015) Use of vancomycin as a surrogate for dalbavancin in-vitro susceptibility testing: results from the DISCOVER studies. Ann Clin Microbiol Antimicrob 14: 19.
- Jauregui LE, Babazadeh S, Seltzer E, Goldberg L, Krievins D, et al. (2005) Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis 41(10): 1407-1415.
- Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, et al. (2014) Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 370(23): 2169-2179.
- Citron DM, Tyrrell KL, Goldstein EJ (2014) Comparative in vitro activities of dalbavancin and seven comparator agents against 41 Staphylococcus species cultured from osteomyelitis infections and 18 VISA and hVISA strains. Diagn Microbiol Infect Dis 79(4): 438-440.
- Darouiche RO, Mansouri MD (2005) Dalbavancin compared with vancomycin for prevention of Staphylococcus aureus colonization of devices In-vivo. J Infect 50(3): 206-209.
- Dorr MB, Jabes D, Cavaleri M, Dowell J, Mosconi G, et al. (2005) Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. J Antimicrob Chemother 55 Suppl 2: ii25-30.
- Scheinfeld N (2006) Dalbavancin: a review for dermatologists. Dental Online J 12 (4): 6.
- Marbury T, Dowell JA, Seltzer E, Buckwalter M (2009) Pharmacokinetics of dalbavancin in patients with renal or hepatic impairment. J Clin Pharmacol 49(4): 465-476.
- Iarikov D (2013) Dalvance Clinical Review. Center for Drug Evaluation and Research.






























