Pharmacognosy in Community Pharmacy: Pharmacovigilance Prospective of Herbal Medicine
Kumud Upadhyaya*
Department of Pharmaceutical Sciences, Kumaun University, India
Submission: April 27, 2017;Published: June 01, 2017
*Corresponding author: Pugazhendy K, Department of Zoology, Annamalai University, India, Email: upkuupku@gmail.com
How to cite this article: Kumud Upadhyaya.Pharmacognosy in Community Pharmacy: Pharmacovigilance Prospective of Herbal Medicine. 005 Glob J Pharmaceu Sci 2(4) : 555593 (2017). DOI: 10.19080/GJPPS.2017.02.555593
Abstract
A recent report suggests global market for medicinal plants is around $60 billion growing at a brisk pace of seven to eleven percent annually.Out of the annual consumption of raw drugs, 50 per cent are from roots, 15 per cent from fruits/seeds, 12 per cent from wood, 9 per cent from whole plants, 7 per cent from bark/stem, 4 per cent from leaves and 3 per cent from flowers. Termed as CAM (Complimentary and Alternative Medicine) herbal medicine have long been considered safe alternative for modern medicine. Recent development in research in herbal medicine has necessitated the efforts to further explore safety aspects of Herbal medicine. Pharmacovigilance of herbal medicine is now an issue to be given ample attention. FDA across the word has tightened their noose on safety issues of herbal medicine.
Keywords: Pharmacovigilance; Safety; CAM; Adverse drug reactions; Quality assurance
Abbreviations: DSHEA: Dietary Supplement Health Education Act; CAM: Complementary and Alternative Medicines; HMPs: Herbal Medicinal Products; OTC: Over The Counter; CDSCO: Central Drugs Standard Control Organization; PvPI: Pharmacovigilance Programme of India; ADRs: Adverse Drug Reactions; NCC: National Co-ordination Centre; AMCs: ADR Monitoring Centers; PV: Pharmacovigilance; ICSRs: Individual Case Safety Reports; PIDM: Programme for International Drug Monitoring ; GMP: Good Manufacturing Practices
Introduction
On February 6, 2004, FDA issued a final rule stating that supplements containing "ephedra" present an unreasonable risk of illness to those taking them. This rule puts ban on manufacture and distribution of ephedra containing dietary supplements with effect from April 12, 2004. This was the first time in US that a supplement has been removed from the market under DSHEA (Dietary Supplement Health Education Act). This was an early warning to the pahrmacognocists world over. It is generally believed that the herbal medicines are safe and posses practically no side effect [1]. But in recent times pharmacognocists have become aware of the fact that much safety data is required for these complementary and alternative medicine. Many of herbal therapies fall under one single title, "Complementary and Alternative Medicines (CAM)."
Every year billions of dollars are spent on herbal remedies. The growth had been phenomenal to the effect of 15-17% in value [2]. Medicinal plants based activities are playing important role in determining global economy. The global market of herbal drugs has registered a steady increase in recent years. The WHO in 1980 estimated world trade of US $500 million [3]. In 2002 the size of drug market was estimated to be approximately around 10 billion US $ with India’s share being only five percent [4,5]. It has now increased to over US$ 14 billion and by year 2050 it may touch US$5 trillion [6]. Lange estimated in 1997 that the Medicinal related plant trade in India was around $80 million per year while the global trade in medicinal -plants was estimated to be over $60 billion per year [7]. The world trade figures suggest that India ranks next to China in annual export (32,000 tones, US $46 million) of medicinal raw material. India's herbal plant trade annual growth rate is of seven percent [8].
Herbs in India
India has six agro-climate zones with 45,000 different plant species and 15,000 medicinal plants. Of these 7000 plants are being used in Ayurveda 700 in Unani, 600 in Sidhha, and 30 in modern system of medicine [9]. Fifty sixth world health assembly of WHO held in March 2003 at Geneva has mentioned that in India 65% of the population in rural area use ayurveda and medicinal plants to help meet their primary needs [10]. It is believed that of total world population 30% of dicotyledonous plants are found only in contrast to China, which has mostly the diploid. Polyploidy results in diverse yield of active constituents [11]. A critical review of Indian Himalayas suggests the presence of over 1748 of Indian plant species (angiosperm 1685, gymnosperm 12 and pteriodophytes 51 of medicinal value [12]. These Himalayan plants are largely being utilized in two ways:
(i) domestic consumption by local inhabitants
(ii) Preparation of plant based drugs by pharmaceutical industry [13].
HMP and the world
In some industrial countries like Germany, herbal medicinal products (HMPs) have a long tradition in professional therapy and in self-medication [14]. In 1988 a survey of the complementary and alternative medicine (CAM) among more than 5000 adults in England reported that almost 20% of the sample had purchased over the counter (OTC) herbal medicinal product (HMP) in the previous year. In addition, almost 1% had consulted a herbal practitioner [15]. Similar studies in USA have indicated that the proportion of adults, who had self-treated with herbal medicine and those who had consulted a herbalist, had increased significantly during the period 1990-1997 [16]. In both Western Europe and USA, consumers spend in the range of US$/Euro 1billion per year on HMP. The trend is increasing.
The UK market for herbal medicines was estimated to be almost £65 million in 2000, an increase of 50% over the period of five years, which compared with many other European countries, is rather low. In France and Germany, the two major European markets, retail sales of HMP totalled US$ 2.9billion in 1997. Annual retail sales of 'botanicals (herbal medicine) in the USA were almost US$ 4billion in 1998 [17]. When one compares, that the plant-derived steroids alone account for about 15% ($22billion) of the $150billion world pharmaceuticals market, that the annual market for taxol was estimated to reach $ 1 billion by the year 2000, that the antineoplastic agents vincristine and vinblastine have sales amounting to $100 million per year, that the market for psyllium seed products amount to some $300 million annually and that nicotine and scopolamine patches now have a combined sales of more than $1 billion per year, it is obvious that natural products continue to play important economic as well as therapeutic role in modern medicine [18]. Of the World's twenty-five best selling drugs/ pharmaceuticals, twelve are natural product derived. This includes potent ACE inhibitor like Enalapril that ranks second with a sale of $ 1745 million [19,20].
Over the years, herbal medicines have become popular in the western world, partly due to disenchantment with modern synthetic drugs. Even in the allopathic medicine, substances derived from higher plants constitute 25% of the prescriptions. The WHO has also appreciated the importance of medicinal plants for public health care in developing countries and evolved guidelines to support the member states in their effort to formulate national policies on traditional medicine [21]. Since the herbal medicine is a complex mixture having complicated interaction of compounds, synergy may be expected to play part.
Synergy was a world loosely defined in natural products. In was observed that extracts were more potent then the purified plant products. Synergy is best defined as the interaction of two or more agents such that combined effect is greater than the sum of expected individual effects. The impact of synergy can be understood with one example. Antimicrobial action of alkaloid berberin has been potentiated by a flavonoid 5' methoxyhydnocarpin (5' MHC), present in the same plant (Berberis spp.) in small amounts. 5' MHC is present in good quantity in another plant, of hydnocarpus species and is identified as multi drug resistance inhibitor [22].
Accordingly, a recent WHO guideline states that herbal medicines should be regarded as "Finished, labelled, medicinal products that contain an active ingredient, aerial or underground parts of plants or as plant preparations. Plant materials also include juices, gums, fatty oil, essential oil or any other substance of this nature. Herbal medicine may contain excepients in addition to the active ingredients. Medicines containing plant material combined with chemically defined active substances, including isolated constituents of plant, are not considered to be herbal medicines"[23].
Herbs and adverse drug reaction
Adverse drug reaction of herbal drugs has been reported on several occasions. Some times this is due to mis-identification [24]. These ADRs may be in the form of Purirtis, Urticaria, rash, Rash erythematous, nausea, vomiting, diarrhea, fever, abdominal pain and dyspnoea. The most common reported critical terms for ADRs on herbal drugs are face oedema, hepatitis, angioedema, thrombocytopenia, hypertension, chest pain, convulsions, purpura and dermatitis [25].
Mucuna pruriens is used for patients suffering from Parkinson's disease. The adverse effects are mild and are mainly gastrointestinal in nature. In Parkinsonism, the average dose of atropine is 0.5mg. The effect of the same is toxic in most cases of 10mg or more [26]. Some other drugs which have shown ADR include Digitalis (Cardiac arrhythmias), Podophyllum (Podophyllum poisoning), Aconitum (cardio toxicity) etc. One of the major causes of adverse events is directly linked to poor quality of herbal medicines including raw medicinal plant material, and to the wrong identification of plant species. Cultivating, collecting and classifying plants correctly are therefore of the utmost importance for the quality and safety of products [27].
Authentication and standardization
The single most important factor that stands is the way of wider acceptance of traditional herbal medicine is the inadequacy of standards for checking their qualities by chemical or bioassay method. This also prevents modernization of the method of their preparation or production as there is no way to establish the equivalence of the product made by the method with the original product [28]. According to GMP for herbal drugs, general specification should carry morphological and microscopical description in addition to assay method, limit test for moisture and potentially toxic elements and contaminants e.g. lead, mercury, arsenic etc, admixed foreign materials, adulterants and fungal/microbial contamination and micotoxin should be incorporated [29].
Historically, the botanical emphasis in Pharmacognosy started in early 1990s out of a need to establish the identity of an adulterant in plant drugs. Initially the pharmacognosists were engaged in their pursuit with the aid of five senses, reinforced some times with the microscope and a few chemical color reactions [30]. Collection, proper botanical identification and drying are important initial stages in the development of herbal drugs. Although botanical identification of herbal drugs has been achieved largely, there is still confusion in respect to some drugs. Ayurveda refers the plants used, by their Sanskrit names and there are instances where the same name is applied to two or three different plants. The term Sankhpushpi connotes resemblance of its flower to that of conch shell. There seems to be a lot of confusion in correlating the terms 'vishnukranti' 'shankhpushpi' 'aparajita' etc. to their respective botanical source [31].
Thus, 'Shankhpushpi' is equated with one or other of the following plants depending on the regions in India. Canscoradecussata, Evolvulus alsinoides and Clitoria ternate [32]. Pharmacognostic evaluation is based on wide and varied features. Earlier, it was based on morphological characters such as composition, venation, margin, apex base, surface, outline (shape), color, texture, size odour and taste [33].
The concept of standardization is relatively recent, but is rapidly becoming essential. It can be defined as the establishment of reproducible pharmaceutical quality by comparing a product with established reference substance and by defining minimum amounts of one or several compounds or groups of compounds [34]. Several monographs are now available on quality assurance, authentication and standardization of herbal drugs.
Authentication and standardization is based on following three aspects [35,36].
- Morphology / Microscopical features.
- Physico-chemical Analysis
- Chemical analysis
Biological Activity
I. Morphological/anatomical features: Morphology is the first step towards the authentication of herbs. Plant parts such as flower, fruits, seeds, leaves, barks, roots etc. should be carefully studied. In the case of leaves, apex, base, margin surface, arrangement etc. provide valuable information e.g. Digitalis purpurea can be easily distinguished from Digitalis lanata on the basis of margin and base. Cinnamon barks are compound quill [37].
Plant anatomy is a subject of great importance both from the practical as well as theoretical point of view. The role of quantitative microscopy in pharmacognosy is well established [38]. Microscope is the main tool for analyzing botanical material [39]. Microsopical examination may be of whole plant part such as seed, leaf, root stem or it may be the powdered drug. In both the case, valuable information regarding authentication of herbs can be recorded [40]. Unicellular trichomes of senna are important diagnostic feature in entire leaf as well as powder. Cannabis has typical glandular hairs [41].
II. Physio-chemical analysis: Various pharmacopoeial standards are available for the physiochemical evaluation. This includes:
- Moisture content
- Foreign matter
- Total ash content
- Acid insoluble ash content
- Water soluble extractives
- Alcohol soluble extractives and
- TLC
Also included in physico-chemical features are chemical test for the presence of various classes of constituents such as alkaloids, glycosides, tannins etc. These tests are also termed as color test [42].
Ash values are helpful in determining the quality and purity of a crude drug, especially in powdered form [43]. When vegetable drugs are incinerated, they leave behind an inorganic ash. The ash that is left contains only mineral elements and is called plant ash [44]. The ash in many cases varies within wide limits (e.g. rhubarb: 8-40 percent) and is therefore of little value for purpose of evaluation. In other cases (e.g. peeled and unpeeled liquorice) the total ash figure is of importance and indicates to some extent the amount of care taken in the preparation of the drug [45]. In the determination of total ash value, the carbon must be removed at as low temperature (450 °C) as possible, since alkali chloride that may be volatile at high temperature would otherwise be lost.
The total ash may then be treated with dilute hydrochloric acid to determine the percentage of acid insoluble ash. Calcium oxide or carbonate, yielded by incinerated oxalate, is soluble in hydrochloric acid. The weighed residue then provides acid insoluble ash. This usually consists of silica and high acid insoluble ash in drugs such as senna, cloves, liquorice, velerian and tragacanth indicates contamination with earthy materials. Senna leaf, which may be used directly as powdered drug is required to have a low acid insoluble ash (2%). In case of ginger, a minimum percentage of water-soluble ash is demanded. This being designed to detect the presence of exhausted ginger [46].
The solvent extractive values (water-soluble and ethanol soluble extractive values) are used as a means of evaluating drugs. The use of a single solvent can be the means of providing preliminary information on the quality of a particular drug sample. For example in a drug where the extraction process for the constituents commences with water as the solvent any subsequent aqueous extraction on the dried residue will give a very low yield of soluble matter.
Identifying problem areas
It has not long been that Canada banned ayurvedic drugs. High levels of metals in composition were cited as major health risk. Apart from this, there should be special programmes for the pharmacist to train them in dispensing of these herbo pharmaceuticals. The Central Drugs Standard Control Organization (CDSCO) in India launched a National Pharmacovigilance Programme of India (PvPI) in the year 2010 to monitor the Adverse Drug Reactions (ADRs). At present 150 ADR Monitoring Centers (AMCs) are functioning under National Co-ordination Centre (NCC) to monitor and report ADRs across the country. ADR monitoring centers under Pharmacovigilance Programme of India (PvPI) have been established in all the states in India [47].
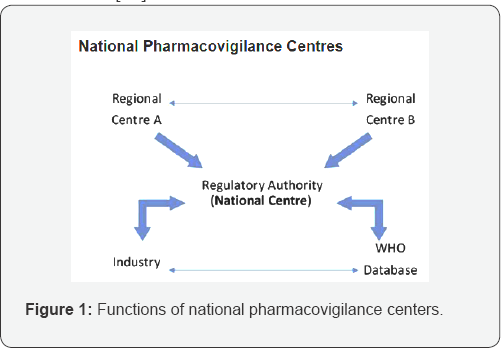
National Centers (NCs) are WHO-approved Pharmacovigilance (PV) centers in countries participating in the WHO Programme for International Drug Monitoring. NCs are usually a part of or closely linked to the national drug regulatory agency. Healthcare professionals and patients (in some countries) send individual case safety reports (ICSRs) to a regional PV centre or a NC. The latter forwards the reports to the central WHO Global ICSR database, Vigi Base that is managed and maintained by the UMC. Every year representatives from the NCs meet and exchange common problems and solutions for promoting medicines safety [48] (Figure 1).
WHO defines Pharmacovigilance (PV) as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. The history of Pharmacovigilance goes back nearly 50 years. In 1965, the 18th World Health Assembly resolution (WHA 18.42) drew attention to the need for adverse drug reaction monitoring. Further resolutions in 1966, 1967 and 1970 resulted in the WHO Programme for International Drug Monitoring (PIDM). Initially, the WHO Programme for International Drug Monitoring (PIDM) consisted of 10 countries as its members. As of October 2015, 122 countries are full members in the programme and contribute to the WHO Global Database, VigiBase. The WHO Collaborating Centre for International Drug Monitoring, the Uppsala Monitoring Centre manages and maintains VigiBase on behalf of WHO and its Member States. There are over 11 million reports of adverse reactions in VigiBase [49]. An adverse drug reaction (ADR) is any noxious, unintended and undesired effect of a drug, which occurs at a dose used in human for prophylaxis, diagnosis, therapy or modification of physiological function [50].
Quality assurance and control measures, such as national quality specification and standards for herbal materials, good manufacturing practices (GMP) for herbal medicines, labelling, and licensing schemes for manufacturing, imports and marketing, should be in place in every country where herbal medicines are regulated. These measures are vital for ensuring the safety and efficacy of herbal guidelines on safety monitoring of herbal medicines in Pharmacovigilance systems medicines. Weak regulation and quality control may result in a high incidence of adverse reactions attributable to poor quality of herbal medicines, in particular resulting from adulteration with undeclared potent substances and/or contamination with potentially hazardous substances and residues [51]. The inclusion of herbal medicines in Pharmacovigilance systems is becoming increasingly important given the growing use of herbal products and herbal medicines globally. A recent study indicated that more than 70% of the German population reported using "natural medicines" and that, for most of them, herbal medicinal products were the first choice in the treatment of minor diseases or disorders.
The worldwide consumption of herbal medicines today is enormous, so that, in terms of population exposure alone, it is essential to identify the risks associated with their use. Safety of herbal medicines is therefore an important public health issue [52]. Despite the growing interest in the safety of herbal medicines, national surveillance systems to monitor and evaluate adverse reactions associated with herbal medicines are rare, even among the more than 70 Member States participating in the WHO International Drug Monitoring Program. The thalidomide tragedy in the late 1950s and early 1960s served as a wakeup call and raised questions about the safety of medicinal products [53]. Pharmacovigilance is therefore one of the important post marketing safety tools in ensuring the safety of pharmaceutical and related health products [54]. Pharmacognocist world over must join heads to avert such tragedy which seems round the corner in absence of proper Pharmacovigilance of herbal medicine.
References
- Martinez HP (1997) Medicinal plants and regional traders in Mexico: physiographic differences and conservational challenge. Economic Botany 51(2): 107-120.
- Olsen GS (1998) The trade in medicinal and aromatic plants from central Nepal to Northern India. Economic Botany 52: 279-292.
- The Hindu Newspaper (2002) India.
- Srinivasan K, Jacob C, Jose J, Jena S (2004) SAJOSPS 4(2): 141-142.
- Mallik AK, Surya K, Chattopadhyay, Falguni (2004) A convenient synthesis of 2-benzoyl-1, 5-diphenylpyrroles, a class of potentially biologically active compounds. Indian J Chem 43(B): 2032-2034.
- Lange D (1997) Medicinal Plants Conservation News Letter 3: 16.
- Holley J, Williams JT (1996) Hindustan Centre of Diversity 12(3): 35,36.
- Indian System of Medicine and Homeopathy in India (2001) Ministry of Health and Family Welfare. Government of India.
- World Health Assembly Provisional Agenda (2003) Geneva, Switzerland.
- Dainik Jagran Newspaper (2001) Sunday Magazine. India.
- Samant SS, Dhar U, Palni LMS (1998) Medicinal Plants of Indian Himalaya: Diversity and Potential Value.
- Purohit AN (1997) Himalayan Biodiversity: Action Plan. In: Dhar U (Ed.), Gyanodaya Prakashan, Nainital, India.
- Eberwin B (2000) Phytomedicine 7 (Suppl II): 43.
- Thomas KJ, Nicholl JP, Coleman P (2002) Use and expenditure on complementary medicine in England: a population based survey. Complement Ther Med 9(1): 2-11.
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, et al. (1998) Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 280(18): 1569-1575.
- Barnes J, Phillipson JD, Anderson LA (2002) Herbal Medicine: A Guide for Healthcare Profession (II edn), Pharmaceutical Press, London, England.
- Robbers JE, Speedie MK, Tyler VE (1996) Pharmacognosy and Pharmacobiotechnology (Rev. Ed.), William and Wilkins, Baltimore.
- Kinghorn AD, Balandrin MF (1993) Human Medicinal Agents from Plants, American Chemical Society, Washington DC, USA.
- Sneaden W (1985) Drug Discovery, Wiley and Sons, Washington DC, USA.
- Venkata RE, Vadlamudi RVSV, Sridhar P (1997) Betulonic Acid And Moronic Acid From The Stem Bark Of Evodia Meliafolia Benth. Visaka Sci J 59(5): 251-253.
- Bhutani KK, Saranjeet Kaur (2003) Organic growth supplement stimulants for in vitro multiplication of Cymbidium pendulum (Roxb.) Sw. Indian J Nat Prod 39(1): 47-52.
- Olayiwola A (1992) Fioiterapia, LXIII (2): 102.
- Saed MA, Ford MR (1999) JMAPA 21:, 1905.
- Rahman SZ, Khan RA, Latif A (2004) Importance of pharmacovigilance in Unani system of medicine. Indian J Pharmacol 40(Suppl 1): S17-S20.
- Patvardhan, Sampada (2004) Pharma Times 36: 31.
- Kusum VD, Devi K, Shaikh SK (2004) Pharma Times 36: 41-45.
- Medicinal Plants-Guidelines to promote Patient Safety and Plant Conservation (2004) Pharma Times 36: 29-30.
- Handa SS (1996) Express Pharma Pulse 21: 32.
- Mallaya M (1996) Express Pharma Pulse 2: 8.
- Handa SS, Kapoor VK (2001) Textbook of Pharmacognosy, Vallabh Prakashan, Delhi, India.
- Sivrajan VV, Balachandran L (1994) Ayurvedic Drugs and Their Plant Source, Oxford and IBH, New Delhi, India.
- Venkata RE (2000) The Eastern Pharmacist, XLIII (509): 35-38.
- Herbert BE, Ellesy KW (1948) Text Book of Practical Pharmacognosy, Billiere Tindall and Cox, London, England.
- (2002) Quality Control Methods for Medicinal Plant Materials, WHO Geneva. (Authorized Reprint), AITBS Publishers and Distributors, Delhi, India.
- The Ayurvedic Pharmacopoeia of India (1989) Ministry of Health and Family Welfare, Government of India.
- The Pharmacopoeial Standards for Ayurvedic Medicines (1986), Central Council for Research in Indian Medicine and Homeopathy, New Delhi,India
- Khandelwal KR (1999) Practical Pharmacognosy, Nirali Prkashan, Pune, India.
- Trivedi PC (2002) Encyclopedia Botanica, Pointer Publishers, Jaipur, India.
- Shome U, Khanna RK, Sharma HP (1980) Pharmacognostic studies onArtemisia scoparia Waldst. and Kit. Proc Indian Acad Sci Plant Sci 93(2): 151-164.
- Gupta RC, Shome U, Khanna RL, Sharma HP (1980) New Botanist VII: 127-143.
- British Herbal Pharmacopoeia (1996) British Herbal Medicine Association, London, England.
- Indian Herbal Pharmacopoeia (1998) IDMA, Mumbai, India, Vol II.
- Kokate CK (1997) Practical Pharmacognosy, (4th edn), Vallabh Prakashan, Delhi, India.
- Kaushik MP (1999) Modern Botany (XII edn), Prakash Publication, MuzaffarNagar, India.
- Wallis TE (1985) Textbook of Pharmacognosy, Ist Indian Edition, CBS Publishers and Distributors, Delhi, India.
- Chaudhry RD (1999) Herbal Drug Industry (Ist edn), Eastern Publishers, Delhi, India.
- http://www.cdsco.nic.in/forms/list.aspx?lid=1578&=1
- http://www.who.int/medicines/areas/quality_safety/safety_ efficacy/nat_centres/en/
- http://www.searo.who.int/india/mediacentre/events/2015/pharma_meet/en/
- Kumar S, Baldi A (2013) Pharmacovigilance in India: Perspectives and Prospects. Journal of Drug Delivery & Therapeutics 3(4): 237-246.
- WHO guidelines on safety monitoring of herbal medicines in Pharmacovigilance systems (2004) World Health Organization, Geneva, Switzherland.
- Shetti S, Kumar CD, Sriwastava NK, Sharma IP (2011) Pharmacovigilance of herbal medicines: Current state and future directions. Pharmacogn Mag 7(25): 69-73.
- Isah AO, Pal SN, Olsson S, Dodoo A, Bencheikh RS (2012) Specific features of medicines safety and pharmacovigilance in Africa. Ther Adv Drug Saf 3(1): 25-34.
- Chan TYK (1997) Monitoring the safety of herbal medicines. Drug Saf 17(4): 209-215.






























