Effect of Oxidative Stress on Sperm Shelterin TRF1 Expression and Chromatin Function in Infertile Males: Preliminary Findings
Anisha John1, Mona Sharma*, Rima Dada2, Reeta Mahey3 , Ashutosh Halder4 and Deepak Pandey5
1MSc, Department of Reproductive Biology, AIIMS, New Delhi, India
2Professor, Department of Anatomy, AIIMS, New Delhi, India
3Professor, Department of Obstetrics & Gynecology, AIIMS, New Delhi, India
4Professor & Head, Department of Reproductive Biology, AIIMS, New Delhi
5Scientist, Department of Reproductive Biology, AIIMS, New Delhi, India
Submission: April 13, 2024; Published: April 30, 2024
*Corresponding author: Mona Sharma, Additional Professor, Department of Reproductive Biology, Room no. 2087, Teaching Blk, AIIMS, New Delhi, India
How to cite this article: Anisha J, Mona S, Rima D,Reeta M, Ashutosh H, Deepak P. Effect of Oxidative Stress on Sperm Shelterin TRF1 Expression and Chromatin Function in Infertile Males: Preliminary Findings. Glob J Reprod Med. 2024; 10(5):555800. DOI: 10.190880/GJORM.2024.10.555800.
Abstract
Oxidative stress has gained more attention as a significant factor in the development of idiopathic male infertility. Infertile men have higher than normal levels of reactive oxygen species in their seminal plasma, and lower levels of antioxidants, which work to neutralize them. Sperm DNA damage is often caused by oxidative stress and is considered a crucial characteristic of semen quality. Telomeres, which are the protective end-caps of chromosomes, are particularly susceptible to oxidative damage that can contribute to telomere attrition especially in infertile males. Shelterin component TRF1 (telomeric repeat-binding factor 1) is involved in the maintenance of the telomere homeostasis. Telomere functions in maintaining chromatin integrity. Therefore, present work aimed to study the effect of oxidative stress on TRF1expression and chromatin function. 10 males were enrolled for this study out of which 5 were normozoospermic (control) males having at least one live birth and 5 were infertile patients (cases) with any one altered semen parameter. Semen was treated with H2O2 to induce oxidative stress in both groups and TRF1 expression was evaluated at mRNA level. The results showed a decrease in the TRF1 expression at mRNA level in the treated group of controls indicating the induction of oxidative stress leading to sperm DNA damage and disruption in telomere homeostasis causing loss in TRF1 expression. In cases, the treated group showed an increased expression of TRF1 which might indicate a compensatory mechanism in response to the DNA damage induced by oxidative stress. In chromatin function analysis, the percentage of decondensed heads decreased in each of the controls and cases after H2O2 treatment indicating the detrimental effect of oxidative stress on chromatin packaging and integrity. Therefore, present study suggests oxidative stress mediated alterations in TRF1 expression and chromatin integrity. But confirmation will be required by more studies conducted with larger cohorts.
Keywords: oxidative stress; shelterin complex; telomeres
Abbreviations: OS: Oxidative Stress; OH: Hydroxyl Radical; ROS: Reactive Oxygen Species; ATM: Ataxia-Telangiectasia Mutated; NHEJ: Non-homologous end joining; TRF1 (telomeric repeat-binding factor 1), TRF2 (telomeric repeat-binding factor 2), POT1 (Protection of telomere 1), TIN2 (TRF1-interacting nuclear factor 2): Rap1: Repressor and Activator Protein 1; DGC: Density Gradient Centrifugation; TPP1: TIN2 POT1 Interacting Nuclear Protein 1; QPCR: Quantitative Real Time Polymerase Chain Reaction
Introduction
Male factor infertility affects one in ten men, and although recent studies have substantially improved the frequency of specific diagnoses, over half of all cases (50–60%) are still classified as idiopathic [1]. In recent years, oxidative stress (OS) has drawn more attention as a significant factor contributing to idiopathic male infertility. OS, which is referred to as a physiological redox imbalance, is brought on by either an excess of reactive oxygen species (ROS) like hydroxyl radical (OH), hydrogen peroxide (H2O2) etc. or a lower level of antioxidants. Leukocytes and immature spermatozoa in the seminal plasma are the major producers of ROS. Despite of highly compacted nucleus in sperm, it is prone for oxidative damage [2]. ROS can cause damage to the sperm nucleic acids like DNA, proteins, and lipids if they are not successfully eradicated by the antioxidant enzymes. Thus, infertile men may have higher ROS concentrations and lower antioxidant levels in their seminal plasma as also reported in study in recent studies. [3,4].
Telomeres are nucleoprotein (Protein-DNA) complexes having variable tandem repeat units of 5′ TTAGGG/CCCTAA 3′ sequences with length ranging between 5 to 15 kb localized at the ends of the linear eukaryotic chromosomes. Telomeres are mainly responsible for protecting the chromosomal ends and maintaining the overall genomic integrity and stability [5]. Telomeric DNA is composed of a 5′-3′ G-rich strand and a 3′-5′ C-rich strand with it’s duplex structure dominating most of its length except at the 3′ end of G-rich strand forming an overhang composed of 35-600 nucleotides [6]. Shelterin, a specialized complex of six proteins, namely TRF1 (telomeric repeat-binding factor 1), TRF2 (telomeric repeat-binding factor 2), POT1 (Protection of telomere 1), TIN2 (TRF1-interacting nuclear factor 2), TPP1 (TIN2 and POT1- interacting nuclear protein 1), and RAP1 (repressor and activator protein 1). Shelterin complex bind to the telomeric DNA and form a lariat-like structure, the Telomeric loop (T-loop), to protect the chromosomal ends from degradation and recognition as damaged DNA.
TRF1 plays a crucial role in regulating telomere length, ensuring effective telomere end capping and facilitating telomere replication. TRF1 exists as a homodimer or high-order oligomer containing multiple DNA binding domains, enabling it to cover a large span of telomeric DNA sequence [7]. TRF1 is known to participate in the repair of double-strand breaks at non-telomeric regions and also in regulating the telomerase activity [8]. The TRF1 subunit of the shelterin complex is involved in inhibiting Non-homologous end joining (NHEJ) and Ataxia-telangiectasia mutated (ATM) signaling pathways, preventing end-to-end chromosomal fusions and maintaining genomic stability [9]. TRF1 has been shown in vitro to loop, bend, and pair telomeric repeats, all of which might promote the telomere folding in vivo [10]. TRF1 along with TRF2 recruits Rap1, TIN2, TPP1 and POT1 proteins to the complex, forming the shelterin assembly.
Telomeres are known to be highly vulnerable to oxidative damage mainly due to their Guanine (G) rich sequences and single-stranded overhangs which leads to telomere attrition. Sperm telomere length shortening is associated with males with idiopathic infertility compared to the fertile men [11]. Therefore, to ascertain the factors contributing to the development of the infertile phenotypes, it is crucial to comprehend the regulation of telomere structure and function. Sperm nuclear chromatin decondensation and male pronucleus formation are the most crucial events that occur during fertilization. Interruptions during the protamination process by which histones are gradually replaced by protamines can lead to abnormal sperm production. This results in male infertility due to increased rates of apoptosis and higher levels of genetic instability [12]. OS may lead to errors in sperm chromatin packaging, resulting in defective chromatin condensation [13]. Considering the independent association of OS, telomere shortening, and chromatin condensation defects with male infertility, the present work aimed to study the impact of OS on sperm shelterin TRF1 expression and chromatin function in infertile males.
Materials and Methods
A total of 10 males were enrolled for this study out of which 5 were normozoospermic (control) males having at least one live birth and 5 were infertile patients (cases) with any one altered semen parameter. The cases were selected from the outpatient Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi. Cases with genital infection, endocrinological disorders, genital tract obstruction, varicocele, chromosomal aneuploidy and anti-sperm antibodies were excluded from the study. Protocol of the study was approved by the institute ethics committee (IECPG- 270/27.04.2022, OT- 06/22.12.2022) and written informed consent was taken from all enrolled cases and controls. Semen samples were obtained from patients referred for baseline semen analysis in the andrology laboratory. Fresh semen sample was collected after 2 to 7 days of abstinence period. A standard semen analysis was performed according to the specifications of the sixth edition of the WHO laboratory manual for the Examination and Processing of Human Semen (2021). One aliquot of the semen samples was used after preparation by density gradient centrifugation for studying the expression of TRF1 both at the basal level and after induction of OS by quantitative real-time PCR (qRT PCR). Another aliquot was simply washed without any preparation technique for assessing the chromatin function both at the basal level and after induction of OS by the nuclear chromatin decondensation assay.
Sperm Preparation by Density Gradient Centrifugation (DGC)
The semen sample was allowed to liquefy for a minimum of 30 minutes at 37°C in the incubator. A double density gradient technique was employed, consisting of 80% ‘lower phase’ and 40% ‘upper phase’. In a 15 ml conical centrifuge tube, 1 ml of the 80% gradient was meticulously pipetted, followed by the gentle layering of 1 ml of the upper phase 40% gradient. Subsequently, 1 ml of the liquefied semen was introduced onto the upper phase layer and the tube was subjected to centrifugation at 350xg for 20 minutes at room temperature. Following centrifugation, the supernatant was carefully removed to avoid disrupting the pellet. The resulting pellet was then resuspended in G-Mops media and subjected to another centrifugation step at 350xg for 10 minutes. After the second centrifugation, the supernatant was discarded, and the pellet was resuspended in 1 ml of G-Mops media. This final suspension yielded a concentrated population of highly motile sperm cells, devoid of any cellular debris such as white blood cells or round cells.
Hydrogen Peroxide (H2O2) Concentration Preparation for Treatment
Oxidative stress on human sperm was induced using H2O2. A thirty percent stock of H2O2 (8.82 M) was diluted to a 10mM H2O2 stock which was used for preparation of 500μM H2O2 concentration.
Total RNA isolation and Quantitative real-time polymerase chain reaction (qPCR)
Extraction of Total RNA from Human Spermatozoa
The resultant sperm suspension after density gradient centrifugation was divided into two equal-volume aliquots, each containing 500μl. One aliquot was subjected to H2O2 treatment of 500μM concentration and both aliquots were incubated at 37°C for 1 hour. Total RNA isolation from spermatozoa was carried out using the RNeasy mini kit (Qiagen) according to the manufacturer’s instructions. Each aliquot containing an appropriate number of sperm cells (not more than 1x107 cells) was pelleted down by centrifuging for 5 min at 300xg in a centrifuge tube. The supernatant was carefully discarded and the tube was flicked thoroughly to loosen the cell pellet. Cells were disrupted and homogenized by adding 600μl of buffer RLT and vortexed for 10 sec. Equal volume of 70% ethanol was added to the homogenized lysate and mixed well by pipetting. Up to 700μl of the sample was transferred to a RNeasy spin column placed in a 2 ml collection tube and centrifuged for 15 sec at ≥8000xg. The flow-through was discarded. Then, 700μl of Buffer RW1 was added to the spin column and centrifuged for 15 sec at ≥8000xg to wash the spin column membrane. The flow-through was discarded. 500μl of Buffer RPE was then added to the spin column and centrifuged for 15 sec at ≥8000xg. The flow-through was discarded. 500μl of Buffer RPE was again added to the spin column and centrifuged again for 2 min at ≥8000xg. The RNeasy spin column was placed in a new 2 ml collection tube and centrifuged at 14000 x g for 1 min to eliminate any residual remains of the buffer and to dry the membrane. The RNeasy spin column was placed in a new 1.5 ml collection tube and 30μl of RNAse-free water was added directly to the spin column membrane and centrifuged for 1 min at ≥8000xg to elute the RNA. The RNA purity and quantity was measured by Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific) and was stored at -80℃ for further use.
Reverse Transcription for cDNA (Complementary DNA) Synthesis
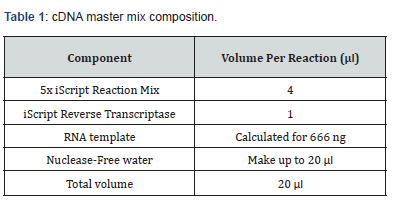
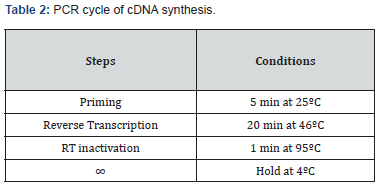
The Reverse Transcription reaction was performed by using iScript™ cDNA Synthesis Kit (Bio-Rad) according to the manufacturer’s protocol. A total of 666 ng RNA was reverse transcribed to obtain cDNA by the use of a blend of oligo(dT) and random hexamers. All the reagents were thawed and prepared on ice (Table 1). The tubes were centrifuged briefly to spin down the contents and eliminate any air bubbles and stored in ice until ready to load in the thermocycler using a specific program (Table 2). The cDNA synthesized was stored at -20°C or was amplified by quantitative real-time PCR using gene specific primers.
Quantitative Real-Time PCR (qRT PCR)
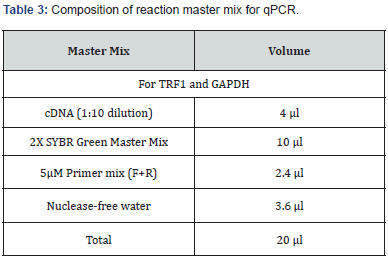
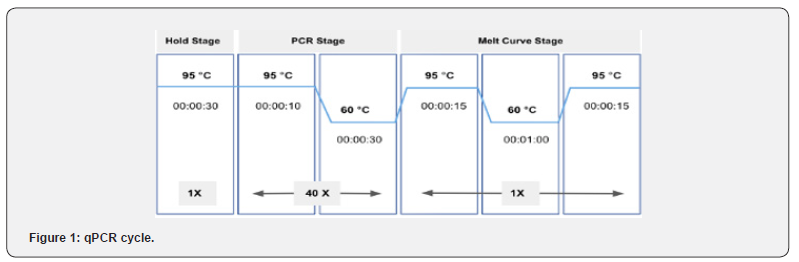
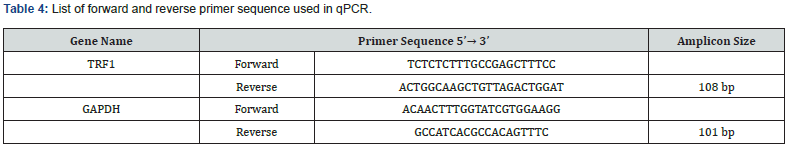
qPCR for the expression of genes at the mRNA level was performed using KAPA SYBR® FAST qPCR Master Mix (2X) Kit (Kapa biosystems) (Table 3). 5 μM concentrations of forward and reverse primers (Table 4) with the specific ratios were prepared separately to be used in the reaction mixture (Table 5). Quant Studio TM 5 (Applied Biosystems TM) Real Time PCR system was used to perform qPCR and the samples were run in duplicate with the specific programmed cycle and melting curve (Figure 1). Ct (cycle threshold) values obtained during real time PCR were used to calculate the relative mRNA expression of the genes. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous control gene for normalizing the fold change. Fold change was calculated using the ΔΔCt method. Relative expression was calculated by the 2-ΔΔCt method.

Nuclear Chromatin Decondensation Test
Semen samples were processed for NCDT before and after the H2O2 treatment. The NCDT was carried out using the NCDT kit (Hi- Tech Solutions) according to the manufacturer’s protocol. Briefly, 100 μl of liquefied semen was taken in each of the two centrifuge tubes, with the second tube treated with 500μM H2O2 and both samples incubated at 37℃ for 1 hour. 1 ml of wash buffer was added to the tubes, followed by centrifugation (5 min at 1500 rpm) and the supernatant was then gently discarded. 200 μl of provided NCD solution was mixed with the pellet, and the tubes were incubated at 37°C for 30 mins. Following this, 50 μl of stop solution was added and incubated for 5 minutes at 37°C. A drop of the mixture was examined under a 40x magnification microscope, with a minimum count of 200 condensed and decondensed heads. Normal percentage was considered to be greater than 70% decondensed Spermatozoa.
Statistical Analysis
The data was analysed using MS Excel and GraphPad Prism 9.5.1 with student t-test and p-value < 0.05 was considered statistically significant.
Results
Semen Analysis
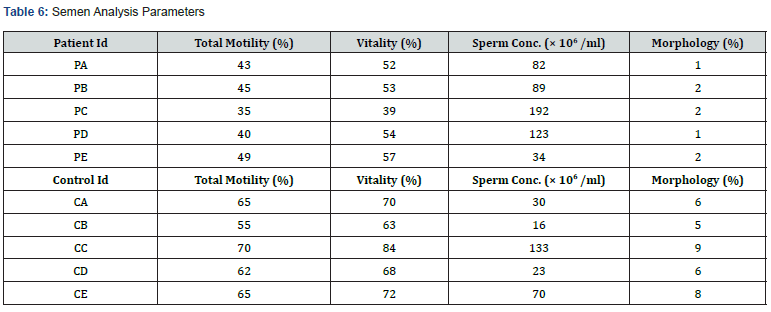
A total of 5 infertile males (cases) and 5 fertile males (controls) were recruited and were instructed for semen sample collection. In cases all five of the ejaculates exhibited any one abnormal semen parameters (mean sperm concentration: 104 × 106 ± 58.55, mean sperm motility: 42 ± 0.053%, mean sperm vitality: 51 ± 0.070%, mean sperm morphology: 2 ± 0.005%) while ejaculates from 5 controls exhibited normal semen parameters (mean sperm concentration: 54.4 × 106 ± 48.68, mean sperm motility: 63 ± 0.055%, mean sperm vitality: 71 ± 0.078%, mean sperm morphology: 7 ± 0.016%) (Table 6).
Quantitative Real Time PCR
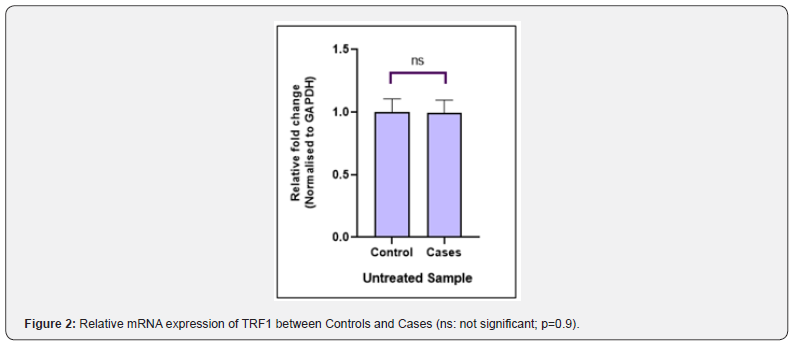
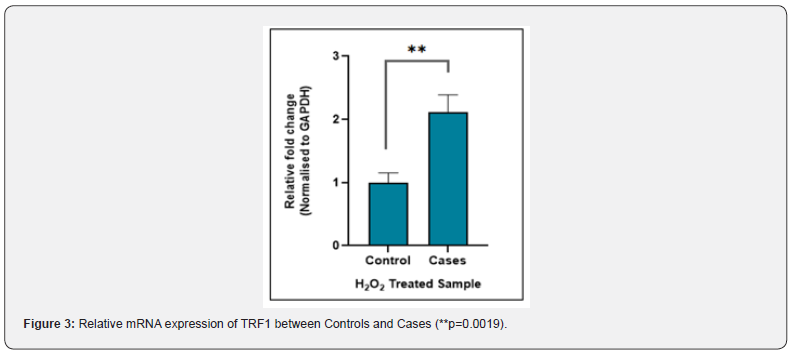
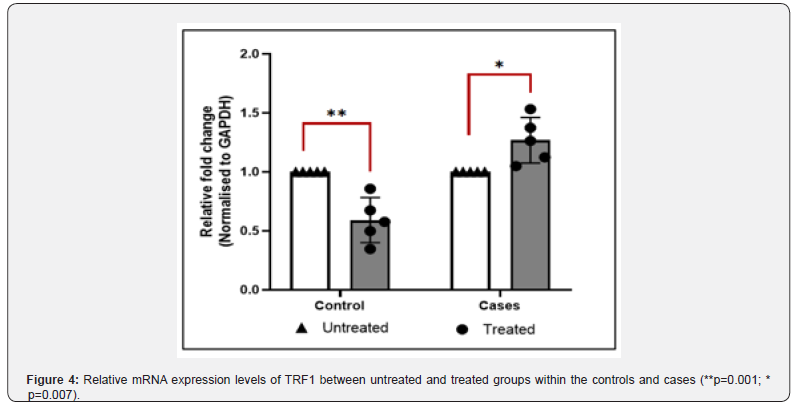
The TRF1 gene expression at the mRNA level was measured by real-time PCR. The relative mRNA expression of TRF1 between controls and cases with and without H2O2 treatment was calculated by using the 2ΔCt method after normalization with GAPDH housekeeping gene. The real time PCR data analysis indicated that the fold change in TRF1 mRNA expression between cases and controls was not significant (P-value = 0.9) in untreated samples (Figure 2), whereas treated samples showed significant difference (P-value = 0.0019) in fold change in TRF1 mRNA expression in cases vs controls (Figure 3). The difference in fold change in TRF1 mRNA expression was found significant in treated vs untreated groups in both controls and cases (P-value = 0.001, 0.007 respectively (Figure 4).
Chromatin Function Assessment - NCDT
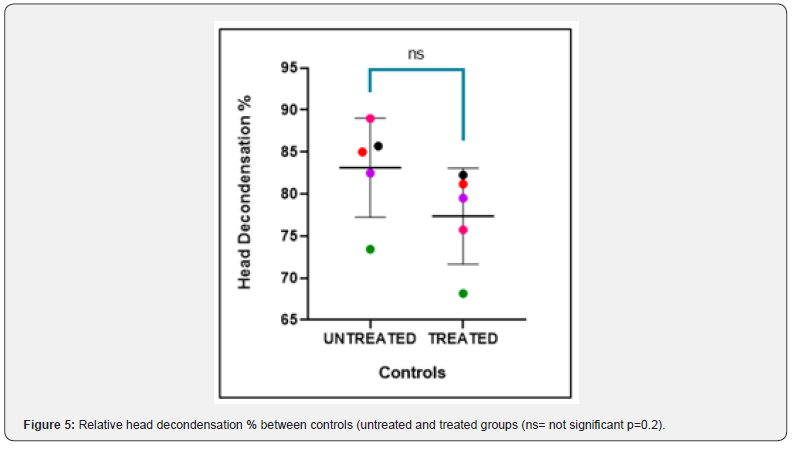
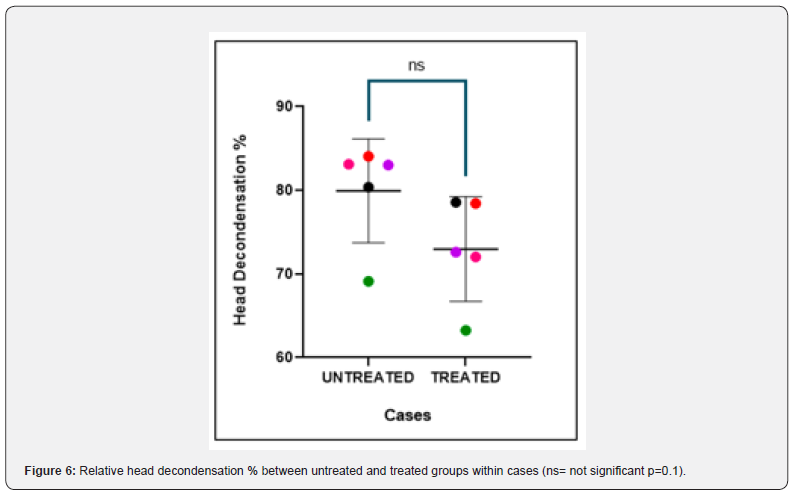
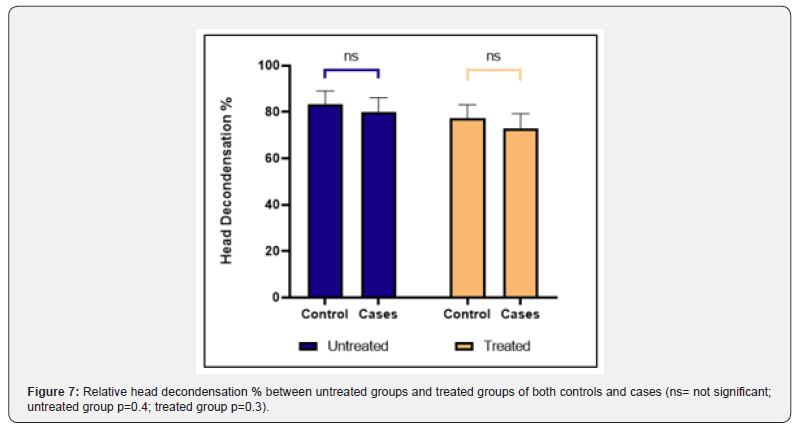
The nuclear chromatin decondensation test (NCDT) was used to assess the ability of the sperm chromatin to decondense after fertilization. More than 70% of spermatozoa that showed decondensed nuclear chromatin were considered as normal. The total count of decondensed heads was composed of both grossly decondensed and moderately decondensed heads. The results of NCDT showed a decrease in the decondensation % in individual controls and cases after H2O2 treatment but the difference was not found to be statistically significant (p value = 0.2, 0.1 respectively) (Figure 5 & 6). The difference in fold change in % of decondensed heads between untreated groups and treated groups of both controls and cases was not found to be significant (P-value = 0.4, 0.3 respectively) (Figure 7).
Discussions
Oxidative stress is a significant factor that leads to sperm cell dysfunction and plays a substantial role in the aetiology of male infertility due to its detrimental impact on both the structural and functional integrity of spermatozoa [14]. Male infertility as well as diminished sperm function have been strongly linked to the degree of sperm DNA damage. ROS damages the DNA of the sperm nucleus by generating base alterations, DNA strand breakage, and chromatin cross-linking [15]. Spermatozoa have limited ability to resist oxidative damage to their DNA, mainly because of the complex packaging of DNA and G rich telomere ends, which are the protective end-caps of chromosomes. Telomere structural changes may also make sperm more susceptible to oxidative stress, which causes fragmentation of DNA and loss of telomere sequences [16]. Thus, impaired telomeric stabilizers like shelterin proteins can be responsible for disrupting the structure of telomeres by affecting the DNA repair pathways as well as chromosomal dynamics [17].
Out of the 5 cases recruited, none of them were oligozoospermic, 60% of them had compromised vitality, 40% of them were asthenozoospermic and all of them showed sperm morphological defects. In controls, normal semen parameters were exhibited. There was no significant difference in sperm count between the cases and controls (p value = 0.2). However, significant differences were observed in sperm motility, vitality, and morphology between cases and controls (p value = 0.0002, 0.0024, and 0.0001, respectively). On qPCR analysis, TRF1 expression was seen to be increased significantly in the cases after H2O2 treatment. This observed increase in the expression of TRF1 may be a response to telomeric DNA damage, functioning as a compensatory mechanism [18]. It has been indicated that oxidative stress, through the introduction of 8-oxoG modifications, has the potential to alter the binding capability of TRF1 to telomeric sequences [19]. Another possible mechanism stated is that TRF1 is a downstream target of ATM following DNA damage. Thus, OS might cause double strand DNA breaks which activates the ATM signaling pathway leading to further phosphorylation of TRF1 and increased expression as observed [20].
In controls, a significant reduction in the expression of TRF1 was observed in the treated group compared to the untreated group. Telomeres are canonical targets of OS thus oxidative damage may lead to telomere attrition and TRF1 protein loss [21]. This might cause DNA damage which could lead to the depletion of crucial proteins responsible for maintaining telomeres. Moreover, oxidative damage induced by H2O2 treatment leads to a high tendency of 8oxoG to be incorrectly paired with adenine during DNA replication, resulting in mutation of the telomeric DNA sequence, which could impede the binding of shelterin components. As a result, this could negatively impact the proper functioning of telomeres. On chromatin function analysis, no significant difference was observed between the decondensation % between the untreated and treated groups of cases and controls. Also, no significant difference was observed between the untreated groups of cases and controls and treated groups of cases and controls. This might be due to the small sample size studied. Reports suggesting that telomere instability reduces gamete viability, ultimately reducing fertility, have risen in prominence in recent years, pointing to telomere homeostasis as a new biomarker in human infertility [22]. There are still unanswered issues about the molecular mechanisms driving male idiopathic infertility and to the best of our knowledge gathered from literature search, no study has been done yet on correlating the oxidative stress associated with male infertility to the shelterin gene expression.
Conclusion
Present work showed the effect of oxidative stress on the TRF1 gene expression and chromatin function in the spermatozoa of infertile males. The findings of the study highlighted that oxidative stress have the potential ability to alter the TRF1 expression by either disrupting the TRF1 binding capability and inhibitory effect of 8oxoG lesion, ultimately causing the loss of TRF1 or driving a compensatory mechanism in response to the DNA damage induced by OS. This damage can contribute to telomere length attrition specially in infertile males, thus studying the regulation of telomere structure and function is essential to identify factors that contribute to the development of male idiopathic infertility. On chromatin function analysis, the percentage of decondensed heads decreased in each of the controls and cases after H2O2 treatment which could indicate the detrimental effect of OS on chromatin packaging. Due to the smaller sample size studied, the difference was not found to be statistically significant. Thus, OS mediated alterations in TRF1 expression and chromatin function is suggestive for present study results. But it is only suggestive until repeated in more studies with larger cohorts.
References
- Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, et al. (2021) Male infertility. The Lancet 397(10271): 319-333.
- Sharma M, Kumar A (2017) The Sperm. In: Basics of Human Andrology, 1st ed, Kumar and Sharma, eds. Springer Nature, Singapore pp. 171-203.
- Mahfouz R, Sharma R, Sharma D, Sabanegh E, Agarwal A (2009) Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertility and sterility 91(3): 805-811.
- Satish PD, Tribhuwan K, Afreen BH, Itagi BN, Naik YK, et al. (2022) Semen Quality in Males Suffering from COVID-19: A Pilot Study. Cureus 14(11): e31776.
- Srinivas N, Rachakonda S, Kumar R (2020) Telomeres and telomere length: a general overview. Cancers 12(3): 558.
- Samassekou O, Gadji M, Drouin R, Yan JU (2010) Sizing the ends: normal length of human telomeres. Annals of Anatomy-Anatomischer Anzeiger 192(5): 284-291.
- De Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes & development 19(18): 2100-2110.
- Yu Y, Tan R, Ren Q, Gao B, Sheng Z, et al. (2017) POT1 inhibits the efficiency but promotes the fidelity of nonhomologous end joining at non-telomeric DNA regions. Aging (Albany NY) 9(12): 2529-2543.
- Schmutz I, de Lange T (2016) Shelterin. Current Biology 26(10): R397-R399.
- Griffith J, Bianchi A, De Lange T (1998) TRF1 promotes parallel pairing of telomeric tracts in vitro. Journal of molecular biology 278(1): 79-88.
- Amir S, Vakonaki E, Tsiminikaki K, Tzatzarakis MN, Michopoulou V, et al. (2019) Sperm telomere length: Diagnostic and prognostic biomarker in male infertility. World Academy of Sciences Journal 1(6): 259-263.
- Gomez M, Wu J, Schreiber V, Dunlap J, Dantzer F, et al. (2006) PARP1 Is a TRF2-associated poly (ADP-ribose) polymerase and protects eroded telomeres. Molecular biology of the cell 17(4): 1686-1696.
- Champroux A, Damon Soubeyrand C, Goubely C, Bravard S, Henry Berger J, et al. (2018) Nuclear Integrity but not topology of mouse sperm chromosome is affected by oxidative DNA damage. Genes 9(10): 501.
- Agarwal A, Virk G, Ong C, du Plessis SS (2014) Effect of oxidative stress on male reproduction. World J Mens Health 32: 1-17.
- Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, et al. (1999) Origin of DNA damage in ejaculated human spermatozoa. Reviews of reproduction 4(1): 31-37.
- Rocca MS, Speltra E, Menegazzo M, Garolla A, Foresta C et al. (2016) Sperm telomere length as a parameter of sperm quality in normozoospermic men. Human reproduction 31(6): 1158-1163.
- Reig Viader R, Capilla L, Vila Cejudo M, Garcia F, Anguita B, et al. (2014) Telomere homeostasis is compromised in spermatocytes from patients with idiopathic infertility. Fertility and sterility 102(3): 728-738.
- Pal D, Sharma U, Singh SK, Kakkar N, Prasad R, et al. (2015) Over-expression of telomere binding factors (TRF1 & TRF2) in renal cell carcinoma and their inhibition by using SiRNA induce apoptosis, reduce cell proliferation and migration invitro. PloS one 10(3): e0115651.
- Opresko PL, Fan J, Danzy S, Wilson DM, Bohr VA (2005) Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic acids research 33(4): 1230-1239.
- Kishi S, Zhou XZ, Ziv Y, Khoo C, Hill DE, et al. (2001) Telomeric protein Pin2/TRF1 as an important ATM target in response to double strand DNA breaks. Journal of Biological Chemistry 276(31): 29282-29291.
- Aeby E, Ahmed W, Redon S, Simanis V, Lingner J (2016) Peroxiredoxin 1 protects telomeres from oxidative damage and preserves telomeric DNA for extension by telomerase. Cell reports 17(12): 3107-3114.
- Reig Viader R, Garcia Caldés M, Ruiz Herrera A (2016) Telomere homeostasis in mammalian germ cells: a review. Chromosoma 125(2): 337-351.






























