Saving the Luteal Phase: The Concept of “Rescue Progesterone”
Sunidhi Minhas*, Santosh Kumar Jena, Reema Basheer, Noushin AM and C Mohammad Ashraf
Department of Reproductive Medicine, Craft Hospital and Research Centre, Kodungallur, Kerala, India
Submission: February 24, 2024; Published: March 18, 2024
*Corresponding author: Sunidhi Minhas, Department of Reproductive Medicine, Craft Hospital and Research Centre, Kodungallur, Kerala, India
How to cite this article: Sunidhi Minhas*, Santosh Kumar Jena, Reema Basheer, Noushin AM and C Mohammad Ashraf. Saving the Luteal Phase: The Concept of “Rescue Progesterone”. Glob J Reprod Med. 2024; 10(5):555799. DOI: 10.190880/GJORM.2024.10.555799.
Abstract
Background: Over the past couple of years, ART has been rapidly evolving with an increase in “freeze-all” cycles causing surge in frozen embryo transfer (FET) cycles worldwide. Artificial preparation using hormone replacement therapy (HRT) is the most convenient method to schedule FET, but in absence of corpus luteum, there is need for an adequate exogenous progesterone to ensure successful implantation and pregnancy. Low levels of serum progesterone(P4) level around the time of embryo has been linked with inferior reproductive outcomes. There is no consensus about cut-off for optimal progesterone level around this time, but use of “rescue protocol” or individualized luteal phase support in case of low serum progesterone may restore reproductive outcomes.
Aims & Objectives: Assess whether a rescue protocol with use of 25 mg subcutaneous progesterone per day can optimize the success rate of HRT FET cycles in women with low serum P4 levels(<12ng/ml) compared to women with adequate serum P4 in terms of β-hCG positive rate(βPR), Implantation Rate (IR), Clinical Pregnancy Rate (CPR), and Ongoing Pregnancy Rate (OPR). Further, comparison considering 7ng/ml as a cut-off for assessing the extent of benefit of rescue protocol.
Materials/patients & Methods: Retrospective cohort study involving data collection from January 2022 to December 2022, where 438 women undergoing HRT FET were included. Results: The IR, CPR and OPR in women with adequate P4 levels undergoing Day 3 embryo transfer were 25.2%, 45.3% and 42.2% and with day 5 embryo transfer were 56%, 69.2% and 59.6% respectively. Whereas, in group with less P4 levels the IR, CPR and OPR in day 3 Embryo transfer were 24.8%, 48.5% and 40.4% and in day 5 embryo transfer were 55.5%, 67.4% and 62.8% respectively. The results showed no difference of clinical significance. Further, considering women with serum P4 < 7 ng/ml who received rescue progesterone, showed reproductive outcomes comparable to that of women with adequate serum P4.
Conclusions: “Rescue protocol” seems to restore the reproductive outcomes in HRT cycles of women with inadequate progesterone levels on the day before transfer.
Keywords: Rescue Protocol; Pharmacokinetics; Progesterone Supplementation; Embryo Transfer
Abbreviations: FET: Frozen Embryo Transfer; IR: Implantation Rate; CPR: Clinical Pregnancy Rate; HRT: Hormone Replacement Therapy; ET: Embryo Transfer; ILPS: Individualized Luteal Phase Support and SC: Subcutaneous
Introduction
In the past couple of years, frozen embryo transfer is being practiced more frequently consequent to a continuous betterment of the cryopreservation facility and almost equal or even better success rate [1-3]. Hormone replacement therapy (HRT) being more simple and flexible method, still remains the most preferred way of endometrial preparation globally. In absence of corpus luteum there is a need for an adequate exogenous progesterone(P4) supplementation to ensure successful implantation as low levels of serum progesterone(P4) around embryo transfer (ET) time has been associated with inferior reproductive outcomes. Serum P4 level around the time of FET has become a hot topic in our current literature where efforts are being made to establish a cut- off value of progesterone, below which the reproductive outcome is compromised. Among the various routes of progesterone supplementation, intramuscular remains the gold standard to achieve maximal plasma concentration of progesterone but micronized vaginal progesterone has always been the heart of progesterone supplementation due to its ease of administration, patient satisfaction and its pharmacokinetics based on uterine first-pass effect, providing better endometrial absorption and more localized action. Some recent reports suggesting aqueous subcutaneous progesterone supplementation can be a good alternative to intramuscular oil-based preparation considering the side effect profile of both [4,5].
In a three-arm randomized control non-inferiority trial, of women undergoing FET in programmed cycle by Devine et al, the arm with only micronized vaginal progesterone had to be prematurely terminated in the interim analysis due to low ongoing pregnancy rate though few other studies didn’t confirm these propositions [6]. In another large prospective cohort study by Vuong et al, which compared vaginal progesterone only group with oral Dydrogesterone and vaginal progesterone combined group for LPS, they concluded that addition of oral Dydrogesterone to vaginal progesterone improves live birth rates and decreases the miscarriage rate [7]. Considering better compliance of vaginal and oral routes, can we individualize and thus optimize the use of progesterone supplementation rather than the blanket use of injectable progesterone in all patients undergoing FET in programmed cycle without compromising the reproductive outcome. The concept of Individualized luteal phase support(iLPS) comes to play a role here, which revolves around use of subcutaneous(s.c.) injection of progesterone administered daily when low serum progesterone is encountered during embryo transfer. There are only a handful studies in literature supporting the use of iLPS, which warrants the need to further investigate into these lines before we incorporate this into our routine clinical practice.
Materials and Methods
Study Design
This is a retrospective cohort study, conducted at Craft Hospital and Research Centre (Kodungallur, Kerala) from 1st January 2022 to 31st December 2022. Ethics committee approval was obtained from from the Institutional Ethics Committee.
Study Population
Eligible patients were women < 40 years’ age undergoing 1st or 2nd ET, with an adequate endometrial pattern (triple layer), with endometrial thickness of >8mm, in Hormone Replacement Treatment (HRT) or down regulated HRT DR-HRT (with use of luteal phase Injection Leuprolide Acetate Depot. 3.75 mg in the previous cycle) FET preparation, after receiving adequate estrogens in proliferative phase. LPS was started with micronized vaginal progesterone 400mg, given 12 hourly with Oral dydrogesterone 10 mg, given 8 hourly for 3 days and 5 days respectively for day 3 and day 5 embryo transfer. Serum progesterone measurements were done one day before ET 4-6 hours after last progesterone dose(vaginal/oral). The results were available the same day, about 2-3 hours after sample collection. As a routine practice at our Centre, if this value was found to be less than 12 ng/ml, aqueous progesterone s.c. formulation of 25 mg was added to the ongoing LPS regimen. LPS was maintained until 12 weeks’ period of gestation or until the day of pregnancy test if negative. We analyzed the pregnancy outcomes of 438 women, who either showed adequate serum P4 levels, i.e., value more than 12 ng/ml and needed no s.c. progesterone formulation in addition to ongoing LPS or inadequate serum P4 levels, i.e., less than 12 ng/ml, where we added the rescue s.c. progesterone injection to ongoing LPS. Exclusion criteria included severe male factor infertility, uterine factor including thin endometrium, uterine anomaly, and endometrial polyps, fibroid (type 0 to 4), sequential embryo transfers and evidence of hydrosalpinx. Serum βhCG level cut off for a positive result is 20 mIU/ml in our institutional set up.
Progesterone Analysis
Blood samples were analyzed by an electrochemiluminescence immunoassay (Cobas e411 analyzer; Roche Diagnostics GmbH, Germany).
Main Outcomes
The primary objective was to compare the reproductive outcomes in terms of beta positive rate(βPR- serum b-hCG levels of >20 IU/mL 14 days after starting the LPS with vaginal and oral progesterone), implantation rate(IR- number of sac implanted per embryo number transferred), clinical pregnancy rate(CPRpresence of at least one intrauterine gestational sac on ultrasound), and ongoing pregnancy rate(OPR- presence of at least one viable fetus beyond week 12) in women with adequate progesterone levels on the day before transfer as compared to women who had inadequate progesterone levels but supplemented with rescue progesterone support. We have further taken into analysis the two subgroups, involving women with P4 less than 7ng/ml as compared to women with P4 in range of 7-12 ng/ml to help assess the degree to which the rescue protocol may alter the above reproductive outcomes.
Statistics
Univariate comparisons between the study and control groups were performed with either the chi-square test or Fisher’s exact test for categorical variables. Independent sample T test was used for comparing baseline characters of the study population. P values < .05 were considered statistically significant.
Analysis made with IBM SPSS software version 23.
Results
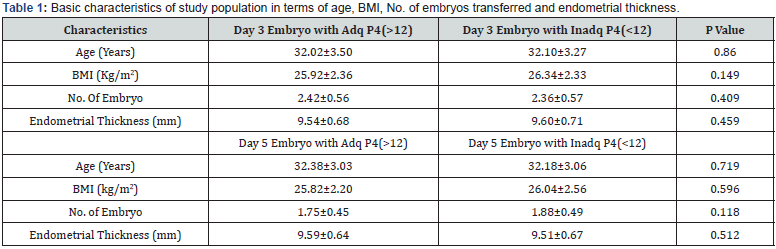
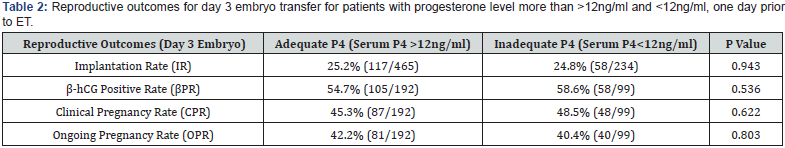
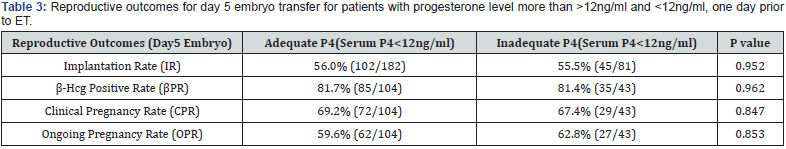
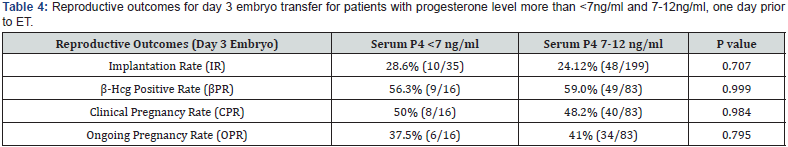
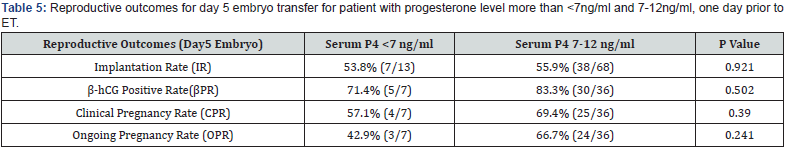
A total of 438 patients were eligible during the mentioned time frame of study for embryo transfer which was performed after artificial endometrial preparation (either HRT or DR-HRT preparation). Overall, the mean serum P4 levels on the day of ET was 17.285ng/ml. Mean serum P4 levels on the day of ET for day 3 and day 5 embryo were 17.140 ng/ml and 17.505 ng/ml respectively. The overall positive b-hCG rate was 56.01%, with an implantation rate of 25.03%, and ongoing pregnancy rate of 41.58% for day 3 embryo. For day 5 embryos beta hCG positive rate was 81.63%, implantation rate was 55.89% and ongoing pregnancy rate was 60.54%. Of the total study population, 142 patients (32.42%) showed serum P levels of less than 12ng/ml and 23 patients showed serum P4 less than 7 ng/ml. All of them were supplemented with s.c. progesterone starting from one day prior to embryo transfer date. Pregnancy leading to live birth was achieved even at low progesterone level of 2.98ng/ml when iLPS was used. The reproductive outcomes in terms of Implantation rate, βhCG positive rate, clinical pregnancy rate and ongoing pregnancy rate was calculated separately for day 3 and day 5 embryos. No difference of statistical significance was found in terms of these outcomes in group with serum P4 >12ng/ml when compared with group with serum P4 <12ng/ml, as can be seen in (Table 1 & Table 2). On further analysis of group with inadequate serum P4 levels, two subgroups were created, one with serum P4<7ng/ ml and second with serum P4 of 7-12ng/ml. The results in these subgroups did not show difference of significance statistically, as can be seen in (Table 3, 4 & 5).
Discussion
This retrospective data analysis was able to determine that patients with low serum P4 levels (performed one day prior to the day of ET) can have similar reproductive outcomes to those with adequate levels when a subcutaneous P4 injection is added to LPS immediately [8]. This finding holds clinical importance, as it facilitates the patient management for women undergoing an ET in HRT/DR-HRT cycles as iLPS can be applied in the midluteal phase without hampering the overall results associated with low serum P4. This is even more relevant for the artificial cycles, as exogenous P4 is added to these cycles to compensate for the absence of a corpus luteum producing endogenous P4. This P4 is crucial for embryo implantation and maintenance of gestation [9], it is of utmost importance to ensure that a minimum threshold is reached to optimize pregnancy rates. This minimum threshold is not very well defined and varies across the literature. A strong correlation exists between serum P4 and various pregnancy outcomes to warrant some intervention in patients with low serum P4 levels [10], where the role of addition of some other of progesterone source to the existing LPS comes into play. Studies in the past have demonstrated that higher doses of vaginal P could decrease the miscarriage rate and improve pregnancy rates [11- 13]. Unfortunately, in these studies’ serum progesterone were not measured, defeating the purpose of correlation.
In our study, we compared reproductive outcomes in the groups and found that addition of s.c. progesterone injection in patients with lower serum P4 values, achieved similar outcomes to that of women with adequate or normal serum P4 levels. Due to ease of use, minimal injection site pain, and the possibility of self-administration as compared with intramuscular injections, the acceptance of s.c. injection was generally high and satisfactory among the patients. A prospective observational study, undertaken by Alvarez et al, published in 2021, also supports the use of subcutaneous P4 injection in patients where low serum P4 was found on the day prior to ET, as this intervention helps to achieve similar reproductive outcomes when compared to those with initial adequate P4 level. The cut off for calling a serum P4 level inadequate was taken as 10.6ng/ml in this study [14]. Yet another study published in year 2021, by Yarali et al, stated that in HRT-FET cycles, addition of 25mg s.c. progesterone injection on daily basis seems to rescue the cycle, by establishing similar reproductive outcomes when compared to patients with adequate serum P4 values, and the cut off for adequate serum P4 level taken by them was 8.75 ng/ml [15]. Labarta et al. also conducted a study, where they have established inferior reproductive outcomes in women with low serum P4 level in an artificial cycle, and further support the concept of iLPS, with addition of s.c. P4 injections to rescue the LPS when the serum P4 level was <9.2 ng/ml [16].
Our study falls in line with the other studies performed in past and mentioned in literature and supports the use of iLPS based on serum P4 level done one day prior or the same day of ET, as this intervention accomplishes similar outcomes to that of women with adequate serum P4 levels, without the need for cycle cancellation. The strength of our study is the good sample size, which includes data with day 3 embryos also, as not all laboratories/IVF centers and patients will have day 5 embryos for transfer. There are a couple of limitations in our study, one being the cut-off taken for serum P4 level. The level is arbitrary and is based on the hospital protocol, and the same cut off has been used for both day 3 and day 5 embryos, where day 3 embryo transfers will have lesser duration of exposure to exogenous P4 in comparison to day 5 though in one of the recent meta-analysis it was shown that progesterone reaches a steady concentration even after 24-48 hours of vaginal progesterone use. Our study is based on an assumption that P4 levels <12 ng/ml causes inferior reproductive outcome which may not be true when both vaginal micronised progesterone and oral dydrogesterone are being used. Also, in our hospital set up, whenever a smaller number of embryos are formed on third day, culture to blastocyst is omitted and embryos are frozen on day 3, making the day 5 embryo transfer cases lesser in number.
Conclusion
“Rescue protocol” seems to restore the reproductive outcomes in artificial cycles of women with inadequate progesterone levels on the day before transfer.
References
- Özgür K, Berkkanoğlu M, Bulut H, Isikli AK (2015) Coetzee Higher clinical pregnancy rates from frozen-thawed blastocyst transfers compared to fresh blastocyst transfers: a retrospective matched-cohort study. J Assist Reprod Genet 32: 1483-1490.
- Wang A, Santistevan A, Hunter Cohn K, Copperman AJ, Nulsen BT, et al. (2017) Freeze-only versus fresh embryo transfer in a multicentre matched cohort study: contribution of progesterone and maternal age to success rates. Fertil Steril 108(2): 254-261.e4.
- Nagy ZP, Shapiro D, Chang CC (2020) Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril 113(2): 241-247.
- Sator M, Radicioni M, Cometti B, Loprete L, Leuratti C, et al. (2013) Pharmacokinetics and safety profile of a novel progesterone aqueous formulation administered by the s.c. route. Gynecol Endocrinol 29(3): 205-208.
- Aflatoonian A, Mohammadi B (2021) Subcutaneous progesterone versus vaginal progesterone for luteal-phase support in frozen-thawed embryo transfer: A cross-sectional study. Int J Reprod Biomed 19(2): 115-120.
- Devine K, Richter KS, Widra EA, McKeeby JL (2018) Vitrified blastocyst transfer cycles with the use of only vaginal progesterone replacement with Endometrin have inferior ongoing pregnancy rates: results from the planned interim analysis of a three-arm randomized controlled noninferiority trial. Fertil Steril 109(2): 266-275.
- Vuong LN, Pham TD, Le KTQ, Ly TT, Le HL, et al. (2021) Micronized progesterone plus dydrogesterone versus micronized progesterone alone for luteal phase support in frozen-thawed cycles (MIDRONE): a prospective cohort study. Hum Reprod 36(7): 1821-1831.
- Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, et al. (2010) Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev 1: CD006359.
- Halasz M, Szekeres-Bartho J (2013) The role of progesterone in implantation and trophoblast invasion. J Reprod Immunol 97(1): 43-50.
- Labarta E, Mariani G, Holtmann N, Celada P, Remohí J, et al. (2017) Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Hum Reprod 32(12): 2437-2442.
- Alsbjerg B, Polyzos NP, Elbaek HO, Povlsen BB, Andersen CY, et al. (2013) Increasing vaginal progesterone gel supplementation after frozen-thawed embryo transfer significantly increases the delivery rate. Reprod Biomed Online 26(2): 133-137.
- Check JH, Dietterich C, Cohen R, Choe JK, Amui J, et al. (2010) Increasing the dosage of progesterone (P) supplementation from the mid-luteal phase in women not attaining a mid-luteal homogeneous hyperechogenic (HH) pattern with sonography improves pregnancy rates (PRS) following frozen embryo transfer (ET). Clin Exp Obstet Gynecol 37(1): 13-14.
- Orvieto R, Meltcer S, Volodarski M, Scharf S, Rabinson J, et al. (2007) Luteal phase support for patients undergoing frozen-thawed embryo transfer cycles–the required progesterone dose. Clin Exp Obstet Gynecol 34(1): 25-26.
- Álvarez M, Gaggiotti-Marre S, Martínez F, Coll L, García S, et al. (2021) Individualised luteal phase support in artificially prepared frozen embryo transfer cycles based on serum progesterone levels: a prospective cohort study. Hum Reprod 36(6): 1552-1560.
- Yarali H, Polat M, Mumusoglu S, Ozbek IY, Erden M, et al. (2021) Subcutaneous luteal phase progesterone rescue rectifies ongoing pregnancy rates in hormone replacement therapy vitrified-warmed blastocyst transfer cycles. Reprod Biomed Online 43: 45-51.
- Labarta E, Mariani G, Rodríguez-Varela C, Bosch E (2022) Individualized luteal phase support normalizes live birth rate in women with low progesterone levels on the day of embryo transfer in artificial endometrial preparation cycles. Fertil Steril 117(1): 96-103.






























