Metformin Reduces the Extent of Varicocele-Induced Damage in Testicular Tissue
Erkan Erdem1*, Nazli Ece Güngör-Ordueri2, Akin Usta3, Cenk Kığ4 and Meric Karacan5*
1Department of Urology, Ota-Jinemed Hospital, Turkey
2Department of Histology and Embriyology, Biruni University, Turkey
3Department of Obstetrics and Gynecology, Balikesir Univesity, Turkey
4Department of Medical Biology and Genetics, Yeni Yuzyil University, Turkey
5Department of Obstetrics and Gynecology, Yeni Yuzyil University, Turkey
Submission: December 31, 2018; Published: February 08, 2019
*Corresponding author: Erkan Erdem, Department of Urology, Ota-Jinemed Hospital, Turkey
How to cite this article: Erkan E, Nazli E G O, Akin U, Cenk K, Meric K. Metformin Reduces the Extent of Varicocele-Induced Damage in Testicular Tissue. Glob J Reprod Med. 2019; 6(4): 555691. DOI:10.19080/GJORM.2019.06.555691.
Abstract
Varicocele is a medical condition where retrograde flow of blood leads to increased hydrostatic pressure in testicular veins and the prevalence of varicocele is predicted to vary between 30 to 40% in infertile men. Metformin, major therapeutic agent in the treatment of type 2 diabetes mellitus, was shown to reduce oxidative stress and apoptosis in renal tubular epithelial cells and testis of animal models. However potential protective effects of metformin against varicocele-induced testicular damage has not been studied. We investigated the impact of metformin on spermatogenesis assessed with Johnsen score, seminiferous tubule integrity and apoptotic activity assessed with the expression of cleaved caspase 3 activity. A total of 36 male Wistar rats (6-week-old) were divided into six groups (n=6 for each group); (C) control group, S (sham group), V (varicocele-only group), V+M (varicocele + metformin group), V/E (varicocele + varicocelectomy group), V/E+M (varicocele + varicocelectomy + metformin group).
Metformin administration improved spermatogenesis, seminiferous tubule integrity and reduced apoptotic activity as manifested by the decreased expression of cleaved caspase 3 in rats with varicocele. However, metformin did not exhibit any additional benefit on these parameters in varicocelectomies rats. As Results, metformin treatment reduced the extent of damage to spermatogenesis in rats with varicocele, although no additional benefit was detected when administered following varicocele surgery.
Introduction
Varicocele is an abnormal vascular dilatation of pampiniform plexus, commonly developing at puberty. Although underlying mechanisms remain poorly understood genetic background, anatomical aberrations, incompetence of venous valves, difference between the drainage of left and right testicular veins were suggested in the etiology [1]. As left spermatic vein being longer than the right vein, it is more commonly incurred to increased hydrostatic pressure and dilatation. Compression of the left renal vein between the aorta and the superior mesenteric artery may also contribute to the disturbed intravenous pressure [2].
The prevalence of varicocele varies between 15-20 % in general population and 30-40% in infertile men, and 11-19% of adolescents [3-6]. It was reported that varicocele is a progressive disease and early diagnosis and treatment in youth may enhance fertility potential [7]. Several contributing factors in the pathophysiology of varicocele have been proposed such as higher temperature of testis, the disorder of neuroendocrine system, autoimmunity, accumulation of renal and adrenal metabolites, genetic and epigenetic factors, hypoxia and oxidative stress [8-10].
Varicocele represents a chronic process within the testicle, which is linked to increased reactive oxygen species (ROS) beyond physiologic limits and, subsequently, disrupting sperm membrane fluidity, causing DNA damage and necrosis [11]. Moreover, superoxide dismutase 1, glutathione S-transferase M1 and T1 which are counteracting free superoxide radicals in cells have been reported to be decreased in men with varicocele, that may be important on disturbed sperm parameters [12]. Apoptosis of germ cells was also demonstrated in the pathogenesis of varicocele-related infertility [13]. Clinical findings suggest that surgical repair of varicocele may decrease seminal oxidative stress levels and sperm DNA fragmentation and, thus, may improve sperm quality [14]. Therefore, surgical intervention seems to be a reliable option in the treatment of varicocele-related male infertility, although some controversial reports exist.
Additionally, anti-oxidant medications such as kallikrein, L-carnitine with L-acetyl carnitine, pentoxifylline, coenzyme Q10 have been used to improve the milieu in the testis in men with varicocele [15]. Metformin is a major therapeutic agent in the treatment of type 2 diabetes mellitus as an insulin sensitizer, which decreases hepatic glucose output and increases peripheral glucose uptake. Although its action was not fully elucidated, metformin attenuated intracellular reactive oxygen species and apoptosis in aortic endothelial cells, myocardium, renal tubular cells and testicular cells [16-20].
Aim
Potential effects of metformin on varicocele-induced testicular damage have not been studied in neither humans nor in animal models. Thus, we investigated the impact of metformin on spermatogenesis, testicular integrity, and apoptotic activity in the testis of adolescent rats with experimentally-induced varicocele.
Materials and Methods
Thirty-six male adolescent Wistar rats (6-week-old) were randomly and equally divided into six experimental groups. Surgical procedures were carried out under anesthesia with intraperitoneal injection of ketamine (50 mg/kg). The experimental groups were as follows:
• (C) Control group; no surgical procedure was performed, and testis was examined after removal.
• (S) Sham group, a midline incision was performed, and testis was examined 8 weeks later.
• (V) Varicocele - only group: Experimental varicocele was induced by partial ligation of left renal vein with
Silk suture at the area medial to the insertion of the adrenal and spermatic vein into renal vein as described previously [21].
• (V+M) Varicocele + metformin group: All rats were treated with metformin (300 mg/kg per day by oral gavages) for 8 weeks following induced varicocele.
• (V/E) Varicocele + varicocelectomy group: Varicocelectomy was performed 4 weeks and the examination of the testis 8 weeks after the induction of varicocele. No medication was used.
• (V/E+M) Varicocele + varicocelectomy + metformin group: Varicocelectomy was performed 4 weeks after the induced varicocele. Metformin treatment (300 mg/kg per day by oral gavages) was initiated after the induction of varicocele and continued for 8 weeks. Left testes were examined 8 weeks after the induction of varicocele in all varicocele - induced groups. As maximum apoptotic activity initiates approximately 28 days after the induction of varicocele the procedure of varicocelectomy was performed 4 weeks after the formation of varicocele [22].
Histologic preparation and evaluation
The testicular tissue was fixed in Bouin’s solution (75% picric acid, 5% glacial acetic acid, and 25% formaldehyde) and embedded in paraffin blocks. Sections (5 μm) were formed, deparaffinized, and stained with hematoxylin and eosin. Spermatogenesis was examined in each group using Johnsen’s score (a score of 1-10 was assigned to each tubule regarding epithelial maturation) as described previously [23]. Sections were examined in a random order under a standard light microscope with 10x and 40x magnification by a blinded histologist; unaware of which group each rat belonged to. Histological grading was done by examining approximately 80 randomly selected seminiferous tubules per rat. Thus, a total of approximately 480 seminiferous tubules were scored for each group.
Histomorphometry analysis
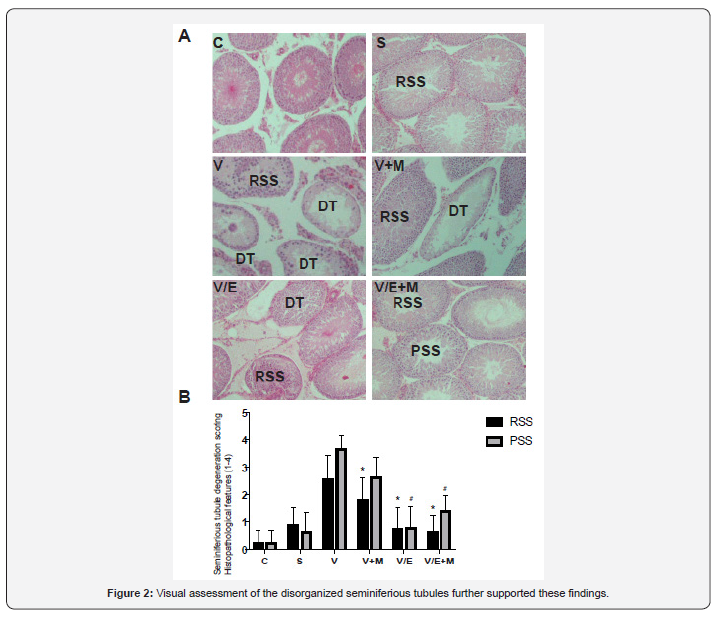
A total of 103 randomly selected seminiferous tubules stained with hematoxylin-eosin were analyzed in each group. The presence of round spermatid stage (RSS) and primary spermatocyte stages (PSS) were assessed as described previously and compared among the groups [24].
Immunohistochemical staining for cleaved caspase-3 and ImageJ analysis
Cleaved caspase-3 was used for immunohistochemical staining. Testicular tissue samples were immediately fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned (5 μm). Sections were deparaffinized and blocked for endogenous peroxidase activity with methanol containing 3% H2O2 for 10 m. Ultra V Block (Lab vision, Freemont, CA) for 7 m at room temperature. Cleaved Caspase-3 (#9664, Cell Signaling, U.S.) was applied at a dilution of 1: 500 and incubated overnight at +4 °C in a humidified chamber for nonspecific binding. The sections were washed in phosphate-buffered saline (PBS) and incubated with biotinylated horse anti-rabbit IgG (3 mg/mL; Vector, Burlingame, CA) at a 1: 500 dilution for 1 h at room temperature.
Antibodies were detected using a VECTASTAIN avidinbiotin complex (Vector PK 4000) for 30 m at room temperature. Antibody complexes were visualized after incubation with 3,3’-diaminobenzidine tetrahydrochloride (DAB, Bio-Genex, San Ramon, CA.) and were mounted under glass coverslips in Entellan (Merck) and then evaluated under a light microscope. Immunohistochemical staining for cleaved caspase-3 was analyzed by counting 100 seminiferous tubule cross-sections in each group and expressed as the apoptotic index. In each photomicrograph, the following parameter was measured with ImageJ software: expression levels of cleaved-caspase-3 in both groups at round spermatid stage (RSS) of testes. Each of this parameter was measured 3 times for each image and the average of the 9 measurements of each sample was used for the statistical analysis. Histopathological features examined in rats with normal testis and with sham, varicocele, varicocele+ metformin in a subjective scoring (0 - not present; 1 - low grade; 2 - moderate grade; 3 - high grade; 4 - very high grade).
Statistical analysis
Histopathological findings (Johnsen’s score) were assessed by nonparametric Kruskal-Wallis test, and the mean Johnsen’s score was used in the comparison of the groups. Multiple comparisons were made using Tukey’s procedure. p<0.05 was considered statistically significant. Analysis of variance was used for statistical analysis of the apoptotic index among the groups.
Results
Assessment of spermatogenesis
Johnsen’s score was significantly lower in V group (4.14±1.25) compared to C group (9.1±0.3) or S group (9.0 ± 0.2) groups (p<0.05). V+M group had significantly higher score (6.9±0.6) than V group (p<0.05). V/E group and V/E+M group had similar Johnsen scores (8.9 ± 1.02 and 9.2 ± 0.6). These findings suggest that the administration of metformin resulted in 40.6% of improvement in spermatogenesis in rats with varicocele. However, this favorable effect was not observed when metformin was used along with varicocelectomy.
Histological and morphological changes in seminiferous tubules
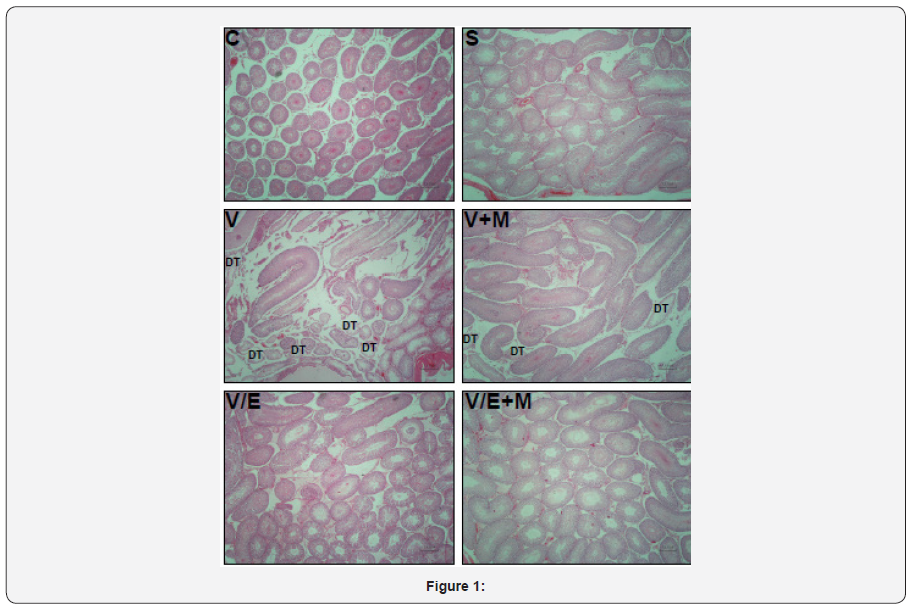
Histological and morphological changes in the testes of rats were compared via hematoxylin and eosin staining and degenerated tubules (DT) were only detected in V and V+ M groups, not in C, S, V/E and V/E+M groups (Figure 1). Visual assessment of the disorganized seminiferous tubules further supported these findings as seen in Figure 2. Seminiferous tubule degeneration scores were used for quantification of data (Figure 2b). V group had significantly higher scores of RSS and PSS compared to C and S group (2.6±0.8 and 3.7±0.4; 0.2±0.4 and 0.2±0.4; 0.9±0.6 and 0.6±0.7, respectively) (p<0.05). V/E group had significantly lower RSS (0.7±0.8) and PSS (0.8±0.7) scores than V group (p<0.05). V+M group had significantly lower RSS and PSS scores (1.8±0.7 and 2.6±0.7, p<0.05) in comparison to V group, implicating beneficial effects of metformin in rats with varicocele. When compared to V/E group, V/E+M group did not exhibit any difference in RSS (0.6±0.6) and PSS (1.4±0.5) scores, suggesting the absence of additive positive effect of metformin in varicocelectomies rats.
Apoptotic activity
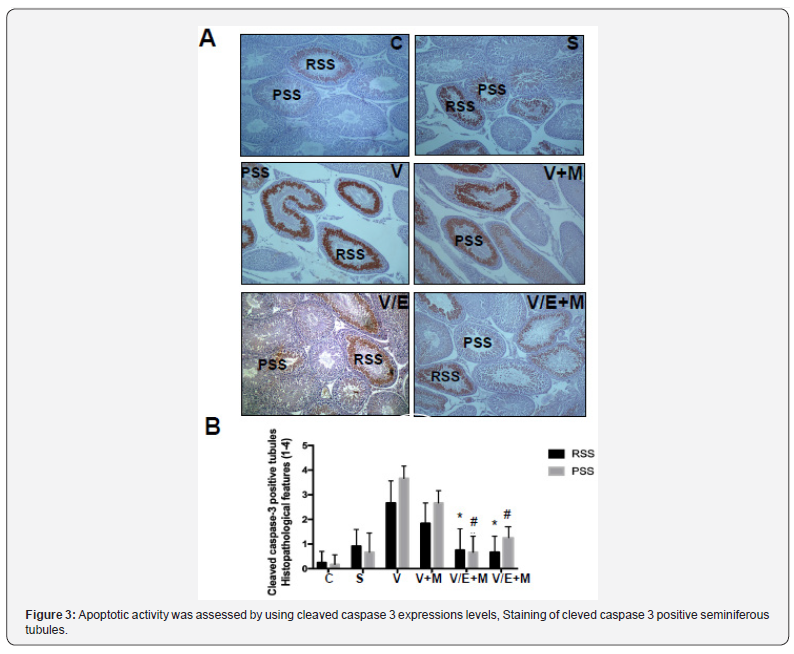
Apoptotic activity was assessed by using cleaved caspase 3 expressions levels, staining of cleved caspase 3 positive seminiferous tubules were shown in Figure 3a. Cleaved caspase 3 expressions were significantly higher in V group (3.5 ± 0.5) compared to C (0) and S (0.2 ± 0.4) groups. V+M group had significantly lower cleaved caspase 3 level (3.0 ± 0.7) than V group. V/E group had lower cleaved caspase-3 expression levels (1.0 ± 0.7) compared to V group. Treatment of varicoceleectomy rats with metformin (V/E+M) did not further reduce apoptotic activity in the seminiferous tubules (1.75 ± 0.43) when compared to the varicocelectomy group (V/E) (Figure 3b).
Discussion and Conclusion
The present study demonstrates that metformin can reduce the extent of testicular damage in rats with varicocele, although having no effect in rats following varicocelectomy Spermatogenesis, seminiferous tubule integrity and the degree of apoptosis were improved using metformin in the presence of varicocele although it was not as remarkable as what was obtained through varicocelectomy. A review of the literature revealed that the impact of metformin on varicocele was not investigated in humans or animal models until now.
Although it is a commonly identified abnormality not all men with varicocele present with infertility. Some intrinsic factors may render some men to become susceptible to varicocele, thus, the best candidates who benefit from varicocelectomy yet to be clarified. Since oxidative stress was shown to be important in the pathophysiology of varicocele some agents have been used to improve the milieu in the testis [1]. A number of anti-oxidant medications have been studied to relieve detrimental effects of varicocele in the testis [25]. These agents have been used either alone or as an adjuvant therapy with surgery. However, surgery remains the treatment of choice and there exists insufficient data to recommend medical therapy in men with varicocele. Barekat et al. [26] reported that administration of an antioxidant agent N-acetyl cysytein as an adjunct therapy improved semen quality following varicocelectomy [26]. Tek et al. [21] demonstrated that vascular endothelial growth factor decreased apoptosis in varicoceleinduced rats as evidenced by diminished caspase-3 positive cells [21]. Both studies showed the benefit of these as adjunct therapy following varicocelectomy. However, in the present study metformin did not enhance the effect of varicocelectomy.
Minutoli et al. [13] demonstrated that neuronal apoptosis inhibin factor and surviving expressions were significantly reduced following varicocele induction and polydeoxyribonucleotide, an agonist of adenosine A2A receptor, administration restored testicular function [13]. Several other studies detected increased germ cell apoptosis in rats with varicocele [21,22,27]. However, in another study, apoptosis was found to be decreased in germ cells in the testes of infertile men with varicocele as compared with normal men [28]. It was speculated that the fixation of testis in formaldehyde might have played a role in the different result. In the present study, cleaved caspase 3 expression was used to assess apoptotic activity and it was found that metformin reduced apoptotic activity in rats with varicocele, whereas no additional effect was observed when metformin was administered after varicocelectomy.
Metformin is commonly used in type 2 diabetes mellitus and polycystic ovarian disease as an insulin sensitizer [29]. Also, it is present in various tissues including myocardium, liver, pancreas, thyroid, adipose tissue, hypothalamus, pituitary, and male and female gonads [19,30,31]. It has been reported that metformin is mainly transported into cells by organic cation transporters as passive diffusion is limited [32]. Although the mechanism of action is not yet fully elucidated recent studies suggested that metformin acts through AMP-activated protein kinase (AMPK) pathway, inhibits the activity of the respiratory electron transport chain in mitochondria, induces epigenetic modifications which in part may explain long term effects and decreases oxidative stress and apoptotic activity [16,19, 33-35].
Male reproductive system utilizes all these metabolic pathways and is prone to be affected by metformin administration [20,36,37]. Metformin was found to stimulate lactate production which is important in the development of germ cells and show an anti-apoptotic effect in rat Sertoli cells [38]. It was also reported that metformin reduced the apoptotic cells and caspase-3 level in rat testis [20]. The findings of the present study are consistent with previous studies that metformin reduced apoptosis in testis with varicocele. Yan et al. [37] reported that metformin improved the semen parameters related to its effects on weight loss, increased testicular weight and reduced testicular cell apoptosis [37]. On the other hand, Tartarin et al. [36] reported metformin at concentration 10 times higher than therapeutic levels decreased testosterone secretion and the number of Sertoli cells in rats when it was administered during pregnancy [36]. Faure et al. [39] reduction in testicular weight and testosterone level were observed in 6-week-old chickens treated with metformin for 3 weeks [39].
Several groups demonstrated that post-operative administration of metformin can exert protective effects in male reproductive function in rat models [40]. Bosman et al. [41] demonstrated that infertile hyperinsulinaemic men could benefit from metformin treatment in combination with an enriched antioxidant diet [41]. Besides, metformin was reported to act as a protective compound when used in the media for cryopreservation of spermatozoa [30]. In conclusion, metformin reduces detrimental effects of varicocele, although no additional benefit is expected following varicocelectomy. Further studies are required to apply metformin for this indication in humans.
References
- Sheehan MM, Ramasamy R, Lamb DJ (2014) Molecular mechanisms involved in varicocele-associated infertility. J Assist Reprod Genet 31(5): 521-526.
- Eisenberg ML, Lipshultz LI (2011) Varicocele-induced infertility: Newer insights into its pathophysiology. Indian J Urol 27(1): 58-64.
- Alsaikhan B, Alrabeeah K, Delouya G, Zini A (2016) Epidemiology of varicocele. Asian J Andrology 18(2): 179-181.
- Levinger U, Gornish M, Gat Y, Bachar G N (2007) Is varicocele prevalence increasing with age?. Andrologia 39(3): 77-80.
- Bogaert GA (2014) Adolescent varicocele: limited indications for treatment during puberty and adolescence. Transl Androl Urol 3(4): 398-401.
- Cayan S, Woodhouse CR (2007) The treatment of adolescents presenting with a varicocele. BJU Int 100(4): 744-777.
- Nork JJ, Berger JH, Crain DS, Christman MS (2014) Youth varicocele and varicocele treatment: a meta-analysis of semen outcomes. Fertil Steril 102(2): 381-387.
- Khera M, Lipshultz LI (2008) Evolving approach to the varicocele. Urol Clin North Am 35(2): 183-189.
- Fretz PC, Sandlow JI (2002) Varicocele: current concepts in pathophysiology, diagnosis, and treatment. Urol Clin North Am 29(4): 921-937.
- Santana VP, Miranda-Furtado CL, de Oliveira-Gennaro FG, Dos Reis RM (2017) Genetics and epigenetics of varicocele pathophysiology: an overview. J Assist Reprod Genet 34(7): 839-847.
- Agarwal A, Hamada A, Esteves SC (2012) Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol 9(12): 678-690.
- Hosseinifar H, Gourabi H, Salekdeh GH, Alikhani M, Mirshahvaladi S, et al. (2013) Study of sperm protein profile in men with and without varicocele using two-dimensional gel electrophoresis. Urology 81(2): 293-300.
- Minutoli L, Arena S, Antonuccio P, Romeo C, Bitto A, et al. (2015) Role of Inhibitors of Apoptosis Proteins in Testicular Function and Male Fertility: Effects of Polydeoxyribonucleotide Administration in Experimental Varicocele. Biomed Res Int 2015: 248976.
- Abdelbaki SA, Sabry JH2, Al-Adl AM1, Sabry HH3(2017) The impact of coexisting sperm DNA fragmentation and seminal oxidative stress on the outcome of varicocelectomy in infertile patients: A prospective controlled study Arab J Urol. 2017 Jun; 15(2):131-139.
- Garg H, Kumar R (2016) An update on the role of medical treatment including antioxidant therapy in varicocele. Asian J Androl 18(2): 222-228.
- Wang ZS, Liu XH, Wang M, Jiang GJ, Qiu T, et al. (2015) Metformin attenuated the inflammation after renal ischemia/reperfusion and suppressed apoptosis of renal tubular epithelial cell in rats. Acta Cir Bras 30(9): 617-623.
- Hou X, Song J, Li XN, Zhang L, Wang X, et al. (2010) reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun 396(2): 199-205.
- Ouslimani N, Peynet J, Bonnefont-Rousselot D, Thérond P, Legrand A, et al. (2005) Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 54(6): 829-834.
- Elmadhun NY, Sabe AA, Lassaletta AD, Chu LM, Sellke FW (2014) Metformin mitigates apoptosis in ischemic myocardium. J Surg Res 192(1): 50-58.
- Ghasemnejad-Berenji M, Ghazi-Khansari M, Yazdani I, Nobakht M, Abdollahi A, et al. (2018) Effect of metformin on germ cell-specific apoptosis, oxidative stress and epididymal sperm quality after testicular torsion/detorsion in rats. Andrologia 50(2).
- Tek M, Cayan S, Yilmaz N, Oğuz I, Erdem E, et al. (2009) The effect of vascular endothelial growth factor on spermatogenesis and apoptosis in experimentally varicocele-induced adolescent rats. Fertil Steril 91(5 Suppl): 2247-2252.
- Fazlioglu A, Yilmaz I, Mete O, Kurtulus F, Parlakkilic O, et al. (2008) The effect of varicocele repair on experimental varicocele-induced testicular germ cell apoptosis. J Androl 29(1): 29-34.
- Johnsen SG (1970) Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1(1): 2-25.
- Tekcan M, Koksal IT, Tasatargil A, Kutlu O, Gungor E, et al. (2012) Potential role of poly (ADP-ribose) polymerase activation in the pathogenesis of experimental left varicocele. J Androl 33(1): 122-32.
- Garg H, Kumar R (2016) An update on the role of medical treatment including antioxidant therapy in varicocele. Asian J Androl 18(2): 222-228.
- Barekat F, Tavalaee M, Deemeh MR, Bahreinian M, Azadi L, et al. (2016) A preliminary study: N-acetyl-L-cysteine improves semen quality following varicocelectomy. Int J Fertil Steril 10(1): 120-126.
- Baccetti BM, Bruni E, Capitani S, Collodel G, Mancini S, et al. (2006) Studies on varicocele III: ultrastructural sperm evaluation and 18, X and Y aneuploidies. J Androl 27(1): 94-101.
- Fujisawa M, Hiramine C, Tanaka H, Okada H, Arakawa S, et.al (1999) Decrease in apoptosis of germ cells in the testes of infertile men with varicocele. World J Urol 17(5): 296-300.
- Naderpoor N, Shorakae S, de Courten B, Misso ML, et al. (2015) Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update 22(3): 408-9.
- Bertoldo MJ, Guibert E, Tartarin P, Guillory V, Froment P (2014) Effect of metformin on the fertilizing ability of mouse spermatozoa. Cryobiology 68(2): 262-268.
- Ferreira C, Sousa M, Rabaça A, Oliveira PF, Alves MG, et al. (2015) Impact of Metformin on Male Reproduction. Curr Pharm Des 21(25): 3621-3633.
- Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, et al. (2008) Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther 83(2): 273-280.
- Zhou G1, Myers R, Li Y, Chen Y, Shen X, et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108(8): 1167-1174.
- Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348(3): 607-614.
- Nguyen L, Chan SY, Teo AKK (2018) Metformin from mother to unborn child - Are there unwarranted effects?. EBioMedicine 35: 394-404.
- Tartarin P, Moison D, Guibert E, Dupont J, Habert R, et al. (2012) Metformin exposure affects human and mouse fetal testicular cells. Hum Reprod 27(11): 3304-3314.
- Yan WJ, Mu Y, Yu N, Yi TL, Zhang Y, et al. (2015) Protective effects of metformin on reproductive function in obese male rats induced by high-fat diet. J Assist Reprod Genet 32(7): 1097-1104.
- Alves MG, Martins AD, Vaz CV, Correia S, Moreira PI, et al. (2014) Metformin and male reproduction: effects on Sertoli cell metabolism Br J pharmacol. 171(4): 1033-1042.
- Faure M, Guibert E, Alves S, Pain B, Ramé C, et al. (2016) The insulin sensitiser metformin regulates chicken Sertoli and germ cell populations. Reproduction 151(5): 527-538.
- Meneses MJ, Sousa M, Alves MG, Oliveira PF (2015) The Antidiabetic Drug Metformin and Male Reproductive Function: An Overview. Int J Diabetol Vasc Dis Res 3(1e) 1-2.
- Bosman E, Esterhuizen AD, Rodrigues FA, Becker PJ, Hoffmann WA (2015) Effect of metformin therapy and dietary supplements on semen parameters in hyper insulinaemic males. Andrologia 47(9): 974-979.






























