Intrauterine Insemination with Fresh Versus Cryopreserved Spermatazoa in Unexplained Infertility
Sheena Rippentrop1*, Randal Robinson1, Tommaso Falcone2 and Gihan Bareh3
1Department of OBGYN, University of Texas Health Science Center at San Antonio, USA
2Department of OBGYN, Cleveland Clinic, USA
3Department of Infertility, Loma Linda University, USA
Submission: June 05, 2018;Published: July 17, 2018
*Corresponding author: Sheena Rippentrop, MD, Department of OB-GYN, San Antonio, TX 78229, 7703 Floyd Curl Drive, USA, Tel: 210-567-50226; Email: rippentrop@uthscsa.edu
How to cite this article: Sheena R, Randal R, Tommaso F, Gihan B. Intrauterine Insemination with Fresh Versus Cryopreserved Spermatazoa in Unexplained Infertility. Glob J Reprod Med. 2018; 5(3): 555662. DOI:10.19080/GJORM.2018.05.555662.
Abstract
Objective: The purpose of this study was to compare pregnancy rates and live birth rates in women with unexplained infertility undergoing ovulation induction and intrauterine insemination (IUI) using fresh versus cryopreserved semen at two institutions.
Methods: Retrospective cohort study conducted at two tertiary care centers via chart review of all women with unexplained infertility who underwent IUI. Other etiologies of infertility were excluded. Pregnancy, clinical pregnancy, and live birth rates were calculated in both groups. Odds ratios and 95 % confidence interval were calculated. A P-value was considered significant when less than 0.05.
Result: A total of 566 IUI cycles were analyzed; 513cycles (90.64%) used fresh semen while 59 cycles (10.42%) used cryopreserved. Clinical pregnancy rates were 29.24% in IUI-fresh and 11.32% in IUI-cryopreserved (OR 3.24, 95% CI 1.36-7.73, p-value 0.0055). The live birth rate per cycle for IUI-fresh was 19.88% versus 3.77% in IUI-cryopreserved (OR 6.33, 95% CI 1.52-26.43, p-value 0.0044).
Conclusion: Significantly higher pregnancy rates and live birth rates were observed when using fresh semen compared to cryopreserved semen in women with unexplained infertility undergoing ovulation induction and intrauterine inseminations.
Keywords: Infertility; Unexplained infertility; Intrauterine insemination; Spermatozoa; Cryopreserved spermatozoa; Female infertility; Fallopian tube; Ovarian reserve; Semen analysis; Clomiphene citrate; Letrozole; Uterine cavity; Mild endometriosis; Polycystic ovarian syndrome; Sperm; Anovulation; Transvaginal ultrasonography; Pelvic exam; Antimullerian hormone levels; Estradiol levels; Chi squared; Fisher’s exact t-test; Transvaginal sono; Ovidrel injection
Abbreviations: IUI: Intra Uterine Insemination
Synopsis
In couples with unexplained infertility, intrauterine insemination with fresh, compared to cryopreserved spermatozoa, results in higher clinical pregnancy and live birth rates.
Introduction
Infertility affects approximately 14% of couples in the general population and is defined as failure to conceive after 12 months of regular unprotected intercourse. Unexplained infertility accounts for up to 15-30% of the diagnoses in couples [1,2]. The diagnosis of unexplained infertility is one of exclusion and can only be made after investigating the common causes of infertility using standard testing including evaluation for male and female infertility [1]. This evaluation typically involves assessment of ovulation, ovarian reserve, fallopian tube patency, uterine and cervical factors, along with semen analysis.
In couples with infertility, previous studies have shown that ovulation induction with intrauterine insemination (IUI) provides a less invasive and more cost-effective treatment option when compared to in-vitro fertilization in couples with unexplained infertility [3]. Therapy is often empiric, as there is no precise cause. Ovulation induction can be accomplished via administration of clomiphene citrate, letrozole, or with injectable gonadotropins. The rationale for ovulation induction with IUI is to increase the number of oocytes available for fertilization and bypass cervical barriers by directly depositing concentrated semen into the uterine cavity [4]. The combination has been shown to be the most effective treatment for infertility compared to timed intercourse, intracervical insemination, or IUI with natural cycle [5]. This method has proven successful in couples with unexplained infertility, cervical factor, mild endometriosis, women with polycystic ovarian syndrome, and couples with mild-moderate male factor infertility [5,6]. Ovulation induction however carries the risk of multiple gestation and thus increasing the obstetrical and neonatal risks including pre-eclampsia, preterm birth, and intrauterine growth restriction [6].
Success of IUI cycles has been shown to be dependent on many factors including maternal, ovulation response, and semen parameters. Improved outcomes have been demonstrated in women of lower maternal age, greater number of pre-ovulatory follicles, and the use of ovarian stimulation for couples with unexplained infertility [7-9]. In regards to seminal qualities, the total motile count is the marker most consistently shown to be a determinant of success in couples undergoing intrauterine insemination [7]. Many have found that sperm preparation techniques in both human and animal models can affect the baseline rate of DNA fragmentation leading to subsequent reduction in both the quality of sperm and fertility rates in samples that have undergone cryopreservation when compared to fresh sperm [10,11]. However, other studies report no difference in pregnancy rates in fresh compared to cryopreserved semen in unselected infertility subgroups [12]. Many studies have evaluated the use of ovulation induction in conjunction with intrauterine insemination in couples with unexplained infertility, yet none have investigated the use of fresh versus cryopreserved semen as a contributing factor in the overall pregnancy rates in the unexplained infertility population. A Medline literature review conducted for the years 1966-2014 did not uncover any previous studies utilizing the search terms unexplained infertility, intrauterine insemination, cryopreserved sperm. Our goal in this study was to evaluate clinical pregnancy rates and live birth rates in women with unexplained infertility undergoing intrauterine insemination with fresh and cryopreserved spermatozoa.
Methods and Materials
This retrospective cohort study examined all women who sought treatment for infertility at the University of Texas Health Science Center at San Antonio and at the Cleveland Clinic for the years 2003-2013. Only patients with unexplained infertility were enrolled. Informed consent was not needed given the retrospective nature of the study and no identifiable data was collected. Exclusion criteria included anyone with known anovulation, polycystic ovarian syndrome, tubal factors, uterine factors, diminished ovarian reserve, male factor infertility, or incomplete records. A total of 566 intrauterine inseminations were performed in 264 women. Of these cycles, 90.64% (513) were performed using fresh semen samples while 10.42% (59) were performed using cryopreserved samples. Patients who chose to use cryopreserved semen were in same sex relationship, did not have a male partner, or their partner was going to be away during the insemination process. Patients ranged in age from 19-46 with an average age of 31.1. Preliminary evaluation of all couples was composed of a full history, physical exam including pelvic exam, transvaginal ultrasonography, assessment of the woman’s ovarian reserve with cycle day 3 follicle stimulating hormone and estradiol levels and/or antimullerian hormone levels, hysterosalpingogram to confirm tubal patency, and semen analysis.
Normal values for these parameters were confirmed in all couples. Prior to intrauterine insemination, all women underwent ovulation induction with clomiphene citrate, letrozole, or injectable gonadotrophins. Clomiphene citrate dosage ranged from 50mg-250mg based on step-up dosing. Letrozole doses ranged from 2.5mg-7.5mg. Injectable gonadotropin dosages varied on a patient to patient basis. Patients were monitored for follicular response using transvaginal sonography with a recruitment goal of 2-3 follicles, each greater than 16mm, however IUI was performed as long as there was at least one dominant follicle present. Some patients however opted out of the monitoring of follicular recruitment, and thus had no ultrasound’s performed. Over-the-counter ovulation predictor kits (urine luteinizing hormone, LH), or choriogonadotropin alfa 250mcg (trade name Ovidrel; Merck; Frankfurter Straße 250, 64293 Darmstadt Germany) trigger shots administered at 24 to 36 hours prior to planned insemination were then used in determining timing of intrauterine insemination. Patients using ovulation predictor kits were told to begin testing on approximately cycle day 10-12 of their cycle depending upon the patient’s menstrual history and medication utilized. The patient’s called on the day the test was positive to schedule intrauterine insemination within 24 hours of a positive LH surge.
Patients unable to detect home LH surges were offered Ovidrel injection when at least two follicles were measured to be greater than 16mm on transvaginal sono. Fresh semen samples were collected on the same day as intrauterine insemination and were prepared using either wash or gradient methods and concentrated to 0.5mL. Following single intrauterine insemination, patients were instructed to take a home urine pregnancy test in 2 weeks if menses had not occurred. If the home pregnancy test was positive, patients were brought into clinic for confirmatory serum pregnancy test and ultrasound assessment at approximately 6 weeks gestation. Retrospective chart review was done for all patients enrolled. Patient data including patient age, ovulation induction method, intrauterine insemination data, clinical pregnancy status, spontaneous abortion, live birth status, and multiple gestation status were recorded from records.
Clinical pregnancy rates, live birth rates, biochemical pregnancy rates, and spontaneous abortion rates were calculated in fresh and cryopreserved insemination groups. Statistical analysis using Chi squared, Fisher’s exact t-test was performed where appropriate and odds ratios and 95 % confidence interval were calculated using Vassar Stats Website for Statistical Computation (vassarstats.net). A p-value was considered significant when less than 0.05. This study was approved by the institutional review boards at the University of Texas Health Science Center at San Antonio and at the Cleveland Clinic.
Result
Clinical pregnancy rates, defined as having a viable intrauterine pregnancy on transvaginal sono, were 29.24% in IUI-fresh and 11.32% in IUI-cryopreserved (OR 3.24, 95% CI 1.36-7.73, p-value 0.0055). The overall live birth rate per cycle for IUI-fresh was 19.88% versus 3.77% in IUI-cryopreserved (OR 6.33, 95% CI 1.52-26.43, p-value 0.0044). Biochemical pregnancy rates in patients who had a positive serum pregnancy test but no intrauterine pregnancy on sono were 12.87% for IUI-fresh and 7.55% IUI-cryopreserved (OR 1.81, 95% CI 0.63- 5.18, p-value 0.2882). Spontaneous abortions, where the patient had a positive serum pregnancy test followed by a confirmed intrauterine pregnancy on sono without a live birth, occurred in 9.36% of IUI-fresh and 7.55% of IUI-cryopreserved cycles (OR 1.26, 95% CI 0.44-3.66, p-value 0.8066). The average age of women undergoing IUI-fresh was 29.7, compared to IUI-frozen average age 35.9. In our study, a multiple gestation rate of 6.82% was observed after any cycle of IUI-fresh (p-value 0.0644). No multiple gestations were seen following IUI-cryopreserved. All multiple gestations consisted of a twin pregnancy, there were no higher order multiples in our study population. All multiples were the result of ovulation induction with clomiphene citrate.
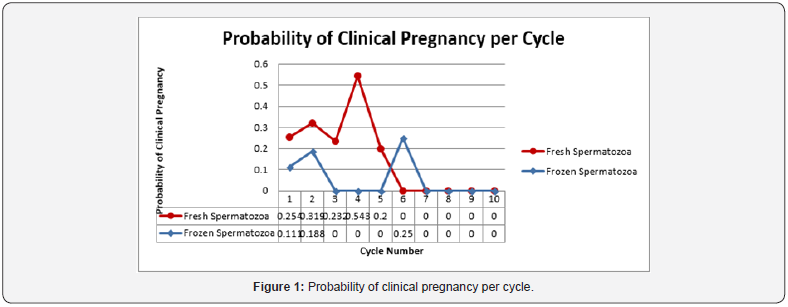
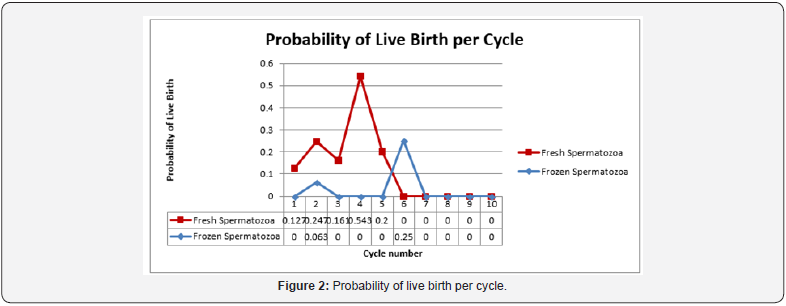
The age of the patient was also determined to be a significant factor in both clinical pregnancy rate and live birth rate. Women under the age of 35 experienced a clinical pregnancy rate of 32.7% compared to women age 35-40 whose rate was 13.1% (OR 3.22, CI 1.83-5.66, p-value <0.0001). Live birth rates were 21.0% and 11.5% respectively for women under 35 and those age 35-40 (OR 2.05, CI 1.12-3.76, p-value 0.0181) No clinical pregnancies or live births were observed in women older than 40 years of age. The vast majority of cycles, 91.7% (519/566) underwent ovulation induction with clomiphene citrate. There were no clinical pregnancies observed in women who used letrozole for ovulation induction, however the study population was too small with only 37 cycles to detect any significant difference. The same holds true for women who chose injectable gonadotropins. There were only 10 cycles of ovulation induction using injectable gonadotropins, which yielded only one clinical pregnancy and one live birth. Again, the data was too small to detect any difference when compared to clomiphene citrate for ovulation induction. Predictably, the probability of both clinical pregnancy and live birth rate declined with each progressive cycle of intrauterine insemination regardless of fresh or cryopreserved spermatozoa when life cycle analysis was performed. The rate of decline when the two were compared was similar however cryopreserved spermatozoa were noted to start at a lower probability than that seen in frozen spermatozoa (Figure 1 & 2).
Discussion
In this retrospective cohort study in couples with unexplained infertility, we found a significantly higher clinical pregnancy rate, and live birth rate for women undergoing ovulation induction and intrauterine insemination using fresh semen compared to cryopreserved semen. No difference was observed in biochemical pregnancy rates or spontaneous abortion rates. We also found that clinical pregnancy rates and live birth rates were significantly higher in this cohort if the woman was under the age of 35. It appears that regardless of the intervention, the most important predictors of success in couples with unexplained infertility remain the age of the woman [1]. Our data supports the addition of fresh spermatozoa to this list of positive prognostic factors, however this observation may be clouded by the fact that the average age of women undergoing IUI-fresh was 29.7 compared to women undergoing IUI-frozen where the average age was 35.9. Unlike the study performed by Wolf et al. [12] but similar to the studies performed by Yildiz et al. [11] and Gosalvez et al. [10] we were able to show a significant difference in pregnancy rates when comparing fresh and cryopreserved spermatozoa [10-12]. These differences are likely due to damage of DNA during the freeze and thaw process of cryopreservation, however this study did not specifically look at semen parameters once male factor infertility had been excluded.
Our finding that women under the age of 35 have better pregnancy rates is consistent with the known decline in overall female fertility after the age of 35. This age-based decline is independent of other factors of infertility. Fecundability, or the ability to conceive per menstrual cycle, has been demonstrated to decline when women are in their early thirties with a more rapid decline around age 35 [13]. Menken et al found that in women aged 31-35 cumulative pregnancy rates begin to decline, and by age 35-39 one-third of women will experience difficulty conceiving [14]. It has been shown to affect not only couples with “normal” fertility but also those undergoing in vitro fertilization and intra-cytoplasmic insemination. Tan et al observed declining fertility rates starting at age 30, and that with increasing age of the woman there was a trend towards lower fertilization rates, clinical pregnancy rates, and live birth rates [15]. These agebased changes are likely due to diminishing ovarian reserve [16], poorer oocyte quality and altered hormone levels resulting in ovulatory dysfunction [17].
Conclusion
Our study suggests that women with unexplained infertility should be counseled that success rates, defined as clinical pregnancy and live birth rate, are higher when fresh spermatozoa is used for insemination compared to cryopreserved spermatozoa. Limitations to this study include different methods and dosing for ovulation induction amongst the study subjects, wide age range of subjects, older data, and the retrospective nature of the study. In future studies, we hope to study prospective, age-matched individuals.
References
- Gelbaya TA, Potdar N, Jeve YB, Nardo LG (2014) Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv. 69(2): 109-115.
- Sakhavar N, Kaveh M, Sadegi K (2014) The impact of letrozole versus clomiphene citrate on uterine blood flow in patients with unexplained infertility. J Family Reprod Health 8(1): 1-5.
- Reindollar RH, Regan MM, Neumann PJ, Levine BS, Thornton KL, et al. (2010) A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril 94(3): 888-899.
- Deaton JL, Clark RR, Pittaway DE, Herbst P, Bauguess P (1997) Clomiphene citrate ovulation induction in combination with a timed intrauterine insemination: the value of urinary luteinizing hormone versus human chorionic gonadotropin timing. Fertil Steril 68(1): 43- 47.
- Ibérico G, Vioque J, Ariza N, Lozano JM, Roca M, et al. (2004) Analysis of factors influencing pregnancy rates in homologous intrauterine insemination. Fertil Steril 81(5):1308-1313.
- Custers IM, König TE, Broekmans FJ, Hompes PG, Kaaijk E, et al. (2011) Couples with unexplained subfertility and unfavorable prognosis: a randomized pilot trial comparing the effectiveness of in vitro fertilization with elective single embryo transfer versus intrauterine insemination with controlled ovarian stimulation. Fertil Steril 96(5): 1107-1111.
- Badawy A, Elnashar A, Eltotongy M (2009) Effect of sperm morphology and number on success of intrauterine insemination. Fertil Steril 91(3): 777-781.
- Duran HE, Morshedi M, Kruger T, Oehninger S (2002) Intrauterine insemination: a systematic review on determinants of success. Hum Reprod Update 8(4): 373-384.
- Veltman Verhulst SM, Cohlen BJ, Hughes E, Heineman MJ (2012) Intrauterine insemination for unexplained subfertility. Cochrane Database Syst Rev 9: CD001838.
- Gosálvez J, Núñez R, Fernández JL, López-Fernández C, Caballero P (2011) Dynamics of sperm DNA damage in fresh versus frozen-thawed and gradient processed ejaculates in human donors. Andrologia 43(6): 373-377.
- Yildiz C, Fleming C, Ottaviani P, McKerlie C (2008) Fresh and frozenthawed sperm quality, nuclear DNA integrity, invitro fertility, embryo development, and live-born offspring of N-ethyl-N-nitrosourea (ENU) mice. Cryobiology 57(2): 156-162.
- Wolf DP, Patton PE, Burry KA, Kaplan PF (2001) Intrauterine insemination-ready versus conventional semen cryopreservation for donor insemination: a comparison of retrospective results and a prospective, randomized trial. Fertil Steril 76(1): 181-185.
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF (1992) Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod 7(10): 1342-1346.
- Menken J, Trussell J, Larsen U (1986) Age and infertility. Science. 233(4771): 1389-1394.
- Tan TY, Lau SK, Loh SF, Tan HH (2014) Female ageing and reproductive outcome in assisted reproduction cycles. Singapore Med J 55(6): 305- 309.
- Speroff L (1994) The effect of aging on fertility. Curr Opin Obstet Gynecol 6(2):115-120.
- Hull MG, Fleming CF, Hughes AO, McDermott A (1996) The agerelated decline in female fecundity: a quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil Steril 65(4):783-790.






























