The Recombinant Vaccine against Human Chorionic Gonadotropin goes on Combined Immunogenicity, Safety and Efficacy Clinical Trials
Talwar GP1*, Radhey S Sarma1, Jagdish C Gupta1, Kripa N Nand1, Bandivdekar AH3, Rohini Sehgal4 and Indrani Ganguli4 Alka Kriplani4
1Talwar Research Foundation, India
2Indian Council of Medical Research, India
3National Institute for Research in Reproductive Health, India
4All India Institute of Medical Sciences, India
Submission: May 15, 2018;Published: July 05, 2018
*Corresponding author: GP Talwar, Talwar Research Foundation, New Delhi, India; Email: gptalwar@gmail.com
How to cite this article: Talwar G, Radhey S S, Jagdish C G, Kripa N N, Bandivdekar A et.al. The Recombinant Vaccine against Human Chorionic Gonadotropin goes on Combined Immunogenicity, Safety and Efficacy Clinical Trials. Glob J Reprod Med. 2018; 5(1): 555654. DOI:10.19080/GJORM.2018.05.555654.
Abstract
A chemically synthesized vaccine inducing antibodies against human chorionic gonadotropin (hCG) was previously developed by Talwar et al. [1] which had undergone successfully Phase I and Phase II efficacy Clinical trials in sexually active women showing its ability to prevent pregnancy without derangement of ovulation,hormonal profiles and menstrual regularity (Table 1). It had high efficacy with only one pregnancy occurring in 1224 cycles. It was fully reversible and women desirous of having another child conceived readily on antibodies falling below 20ng/ml binding capacity [2]. The progeny born to previously immunized women had developmental land marks and cognitive abilities similar to their siblings [3].
Keywords: Vaccine; Antibodies; Sexually active women; Pregnancy; Ovulation; Hormonal profiles; Menstrual regularity; High efficacy; Cognitive abilities; Genetically engineered; Recombinant vaccine; Mycobacterium indicus; Antibody titers; Genetic strains; Non-immunized marmosets; Toxicity; Mammalian Chromosome; Gross pathology; Immunogenicity; Progesterone; Fertility
Abbreviations MIP: Mycobacterium Indicus Pranii; RCGM: Review Committee on Genetic Manipulation; DCGI: Drugs Controller General of India; hCG: Human Chorionic Gonadotropin
Clinical Image
Making of a recombinant vaccine
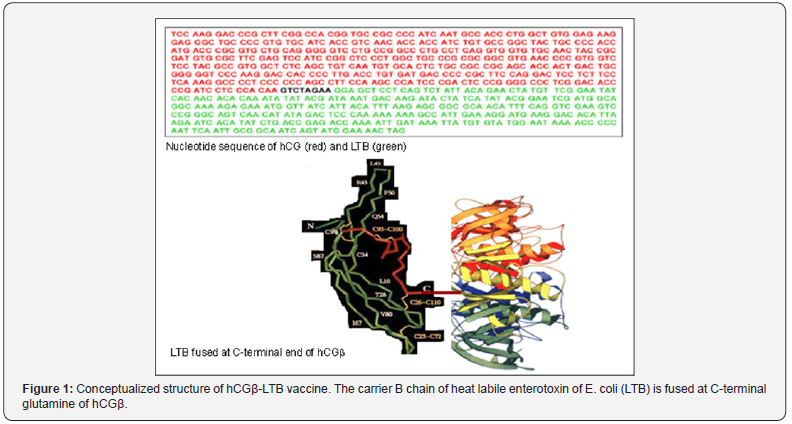
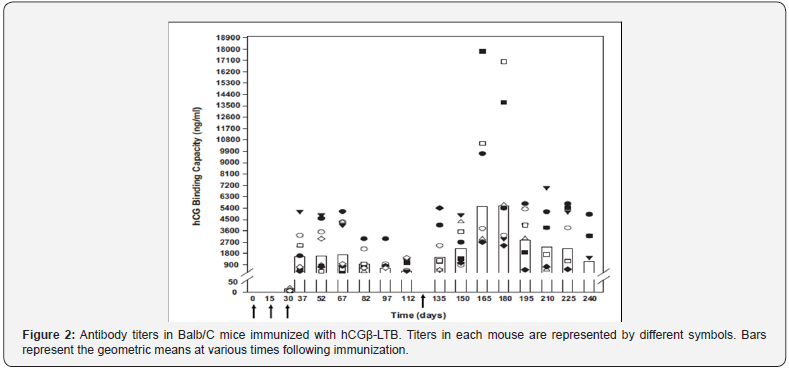
The above mentioned vaccine in the form made in the laboratory was expensive and time consuming. In order to render it amenable to industrial production, we made a genetically engineered version of the vaccine, in which beta subunit of hCG was linked to B subunit of heat labile enterotoxin of E. coli as carrier [4]. Figure 1 shows the sequence of nucleotides of this recombinant vaccine.The recombinant vaccine adsorbed on alhydrogel with adjuvant autoclaved Mycobacterium indicus pranii (MIP), induced fairly high anti-hCG antibodies in not only BalbC, but also in other genetic strains of mice [5,6]. MIP is a potent invigorator of immune response [7]. Figure 2 shows the antibody titersand their duration in BalbC mice. It may be recalled that at 50 ng/ml antibody titers and above, women are protected to become pregnant. This recombinant vaccine was therefore highly immunogenic at least in mice.
Priming with DNA vaccine
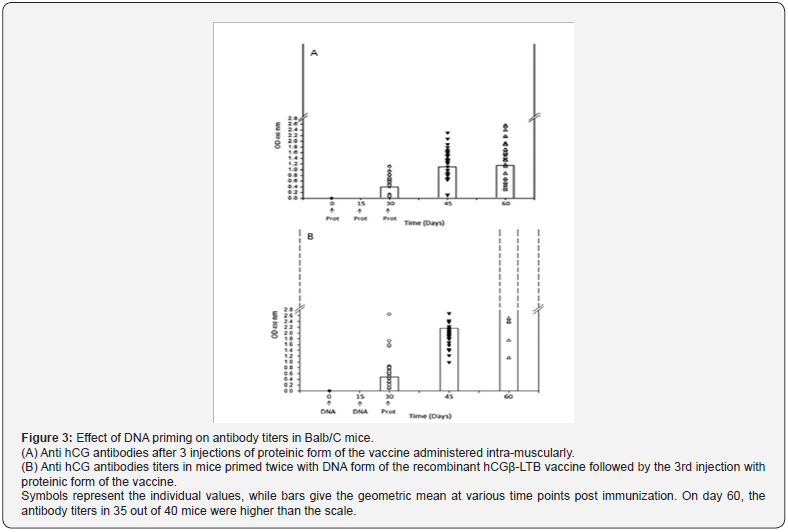
Genetically engineered vaccines are made as both DNA and protein. What was amazing was the observation that if primary immunization is done with the DNA form of the vaccine, followed by injections of the Protein form of the vaccine, the antibody response is substantially higher (Figure 3). Thus to obtain optimal antibody response, the schedule of immunization will be to immunize with 2 primary injections of DNA form of the recombinant vaccine to begin with, followed by third and subsequent injections with the protein form of the recombinant vaccine.
The safety and efficacy of the recombinant hCGβ-LTB vaccine was evaluated in subhuman primate species, the marmosets, at the National Institute of Research in Reproductive Health, Mumbai. The vaccine was found fully safe. 8/9 immunized marmosets were protected from becoming pregnant on cohabitation with fertile males, whereas all non-immunized marmosets in the control group became pregnant.
Toxicology
Being a recombinant vaccine, its approval had to be obtained from Review Committee on Genetic Manipulation (RCGM), the National Committee for recombinant products. They demanded extensive toxicology on this vaccine from an independent GLP company. This was duly done by M/s Bioneeds at their GLP Facility in Bangalore, India. Both DNA and protein forms of the vaccine were non-sensitizing to the skin of guinea pigs with no clinical signs of toxicity, mortality and changes in body weight. Both vaccines were non-mutagenic at the highest concentration tested by Bacterial Reverse Mutation and Mammalian Chromosome Aberration Tests. Similar observation on non-mutagenic property of the vaccines was made in vivo by Mammalian Erythrocyte Micronucleus Test in Mice.Single dose acute toxicity study was conducted in Sprague Dawley rats. Vaccinated rats were observed for mortality, clinical signs of toxicity, body weight and gross pathological examination. No mortality, clinical signs of toxicity and treatment related changes in the body weight, were observed. No changes in gross pathology (external and internal) were observed at even the highest dose tested. Repeat doses of the vaccine were also tested in rats, which were followed up to 90 days post immunization. These studies showed no treatment related changes in physical, physiological, clinical, hematological parameters, as also in histopathology profiles of the organs. Segment II studies in rats showed that vaccines did not affect the embryo-foetal development. Body weight, food consumption, gross pathology remained normal, and no abnormal effect was observed in fetal sex ratio, fetal weight, external, visceral and skeletal norms of fetuses [8] (Table 1).

Approval by RCGM and DCGI
After obtaining the approval of RCGM, we applied for the approval of the Drugs Controller General of India for conducting clinical trials on a protocol which proposed immunogenicity studies at 100, 200, 300, 400 and 500 μg dose of the protein form of the vaccine after priming with 2 doses of 1.5mg of the DNA vaccine. The study will be carried out in 50 women of reproductive age and proven fertility, 10 women at 100 μg, 10 at 200 μg, 10 at 300 μg, 10 at 400 μg and 10 at 500 μg dose of the protein vaccine. A range of parameters will be analyzed before and after immunization. All important laboratory investigations such as anti-hCGtiters, haematological parameters, lipid profile, liver function tests, kidney function tests, serum calcium and phosphorus, total protein, albumin, globulin, progesterone, TSH, prolactin, estrogen, auto antibodies, etc. will be carried out. The trials will be carried out at the reputed institutes, the All India Institute of Medical Sciences, New Delhi and Sir Gangaram Hospital, New Delhi. Subjects will also be clinically examined for weight, blood pressure, androgenism, pelvic TVS ultrasound, pyrexia, H/O joint pains, local reaction at the site of injection, persistent pain at the site of injection and swelling, if any. On completion of these studies satisfactorily, 70 women of proven fertility will be enrolled for efficacy studies. They would be immunized with 2 doses of 1.5 mg of DNA form of the recombinant vaccine at 15 days interval followed by 2 injections of the protein form of the vaccine at the optimal dose determined in earlier phase of the trial. The IUDs will be removed and sexually active women will be observed for a period up to one year for remaining protected against pregnancy during the period, when antibody titers are above 50ng/ml hCG binding capacity.
Acknowledgement
The Project is supported by a grant of the Indian Council of Medical Research.
References
- Talwar GP, Singh O, Rao LV (1988) An improved immunogen for antihuman chorionic gonadotropin vaccine elicited antibodies reactive with a conformation native to the hormone without cross-reaction with human follicle stimulating hormone and human thyroid stimulating hormone. J Reprod Immunol 14(3): 203-212.
- Talwar GP, Singh O, Pal R, Chatterjee N, Sahai P, et al. (1994) A vaccine that prevents pregnancy in women. Proc Natl Acad Sci USA 91(18): 8532-8536.
- Singh M, Das SK, Suri S, Singh O, Talwar GP (1998) Regain of fertility and normality of progeny born at below protective threshold antibody titres in women immunized with HSD-TT vaccine. Am J Reprod Immunol 39(6): 395-398.
- Talwar GP (2013) A Unique Vaccine for Control of Fertility and Therapy of Advanced Stage Terminal Cancers expressing ectopically hCG. Ann NY Acad Sci 1283: 50-56.
- Purswani S, Talwar GP (2011) Development of a highly immunogenic recombinant candidate vaccine against human chorionic gonadotropin. Vaccine 29(12): 2341-2348.
- Purswani S, Talwar GP, Vohra R, Pal R, Panda AK, et al. (2011) Mycobacterium indicus pranii is a potent immunomodulator for a recombinant vaccine against human chorionic gonadotropin. J Reprod Immunol 91(1-2): 24-30.
- Talwar GP, Gupta JC, Mustafa AS, Kar HK, Katoch K, et al. (2017) Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties. Biologics 11: 55-63.
- Nand KN, Gupta JC, Panda AK, Jain SK, Talwar GP (2015) Priming with DNA enhances considerably the immunogenicity of hCGβ- LTB vaccine. Am J Reprod Immunol 74(4): 302- 308.






























