HTLV-1 Associated Adult T cell Leukemia-Lymphoma (ATLL) Presenting as Cutaneous Lymphoma and Hypercalcemia: A Case Report and Review of the Literature
Muhammad Umair*, Amir Shahbaz, Senapathi S, Kashif Aziz, Binita DV, Edward Shalo and Issac Sachmechi
Department of Internal Medicine/Endocrinology, Icahn School of Medicine Mount Sinai, USA
Submission: March 19, 2018; Published: April 12, 2018
*Corresponding author: Muhammad Umair, Resident Physician, Icahn School of Medicine, Mount Sinai, NY, USA, Tel:+l-56-343-6454; Email: muhammadumair0017@gmail.com
How to cite this article: Muhammad Umair, Amir Shahbaz, Senapathi S, Kashif Aziz, Binita DV, etal. HTLV-1 Associated Adult T cell Leukemia-Lymphoma (ATLL) Presenting as Cutaneous Lymphoma and Hypercalcemia: A Case Report and Review of the Literature. Glob J Reprod Med. 2018; 4(2): 555633. DOI: 10.19080/GJORM.2018.04.555633
Abstract
Human T-cell Lymphotropic virus-1 (HTLV-1) belongs to oncovirus family of retroviruses, which unlike HIV virus remains asymptomatic in majority of patients throughout their life time. The latent period of infection varies geographically, ranging from 60 years in Japan to less than 40 years in the Caribbean. The highest incidence and prevalence is seen in Japan (1%-20%), followed by the Caribbean and African countries.An estimated 20 million of population is infected worldwide. HTLV-1 is associated with various diseases such as malignancies, inflammations, opportunistic infections, rheumatic and autoimmune conditions. Hypercalcemia can be due to increased levels of parathyroid hormone related peptide (PTHrP), vitamin D levels, tumor necrosis factor (TNF), transforming growth factor-Beta (TGF-β) and/or interleukins. We present a case of 39 year old male with HTLV-1 associated Adult T cell leukemia-lymphoma (ATLL) presenting as cutaneous lymphoma and hypercalcemia which responded to Zoledronate. The treatment strategy differs from case to case with interferons/chemotherapies for malignancies to antiretroviral medications. However, hypercalcemia management dominates the clinical course.
Keywords: T-cell leukemia-lymphoma; Pneumocystis jiroveci pneumoniae; Tenofovir Alafenamide; Hypercalcemia; Prosultiamin; Pneumocystis jiroveci pneumonia
Abbreviations: ATLL: Adult T-cell Leukemia-Lymphoma; HTLV-1: Human T-Cell Lymphotropic Virus-1; HTLV: Human T-Cell Lymphotropic Virus; ATLL: Aggressive Adult T-Cell Leukemia-Lymphoma; BUN: Blood Urea Nitrogen; PTHrP: Parathyroid Hormone Related Peptide; IV: Intra Venous; TB: Tuberculosis; PJP: Pneumocystis Jiroveci Pneumoniae; PTLV: Primate T-Lymphocytic Viruses; STLV: Simian T-Cell Lymphotropic Viruses; HSM: Hepatosplenomegaly; TGF-β: Transforming Growth Factor-Beta; TNF: Tumor necrosis factor; OCLs: Osteoclasts; HPCs: Hematopoietic Precursor Cells; PCOANs: Phosphonated Carbocyclic 2'-Oxa-3'aza Nucleosides; TAF: Tenofovir Alafenamide
Introduction
Adult T-cell Leukemia-Lymphoma (ATLL) is a highly aggressive malignancy of peripheral helper T-cells as a result of human T-cell lymphotropic virus-1 (HTLV-1) infection. HTLV-1 is a retrovirus belonging to human T-cell lymphotropic virus (HTLV) family that has been associated with several diseases such as aggressive adult T-cell leukemia-lymphoma (ATLL), cutaneous T-cell Lymphoma, myelopathy, uveitis and strongyloides stercoralis infection. Most of the cases are diagnosed after the age of 40 years with a median age of 58 years. An estimated 20 million people are infected worldwide with 1-5% of infected population developing cancer over their lifetime. The highest prevalence is seen in Japan (1-20%), followed by Africa. In United States the general prevalence is 0.1-1% [1]. Here we present a case of ATLL presenting as cutaneous lymphoma and hypercalcemia.
Case Presentation
A 39 year old male presented to Queens Hospital Center with chief complaints of fever, chills and lower extremity pain for one week associated with an unintentional 30 pounds weight loss over a period of four months. He had a past medical history of treatment for chronic infection with H. pylori. During hospitalization basic metabolic profile showed that corrected serum calcium level was 18.4mg/dl (reference range 8.9-10.1mg/dl), blood urea nitrogen (BUN) level was 73mg/dl (7-20mg/dl) and serum creatinine level was 4.88mg/ dl (0.6-1.2mg/dl). On further investigations intact parathyroid (18-64ng/ml) and parathyroid hormone related peptide (PTHrP) level was 2.1pmol/L (normal range <2.0pmol/L). The patient responded to intravenous (I.V.) fluids, calcitonin and zoledronic acid (bisphosphonate derivative and calcium metabolism modifier). Subsequently the patient developed a maculopapular rash, which on biopsy revealed atypical lymphoid infiltrates. The hospital course was complicated by respiratory distress and pulmonary edema. Further testing was done to rule out Legionella, Tuberculosis (TB) and Pneumocystis jiroveci pneumoniae (PJP) infections and they all came back negative, however HTLV-1 antibody testing was found to be positive. The Oncology Department was on board and a diagnosis of Cutaneous T-cell lymphoma secondary to HTLV-1 virus infection along with hypercalcemia was confirmed.
Discussion
For the first time Bernard Poiesz and Francis Ruscetti identified human retroviruses and given the name Human T-cell Lymphocytic Virus (HTLV) at laboratory of National Cancer Institute [2]. The Human T-cell Lymphotropic virus/Human T-lymphocytic virus or human T-cell leukemia-lymphoma virus (HTLV) belongs to a group of human retroviruses which is a sub category of primate T-lymphocytic viruses (PTLV). PTLV has two categories of viruses, those which infect human beings are known as HTLV and those which cause diseases in monkeys are called Simian T-cell lymphotropic viruses (STLV). Both HTLV and STLV have four types as HTLV1-4 and STLV1-4 respectively Furthermore, HTLV-1 has six reported subtypes (subtypes A-F) and its genome is diploid, composed of two single-stranded RNAs. The genome is copied into a double-stranded DNA form via reverse transcription. The infection is thought to spread only through dividing cells as reverse transcriptase generates pro- viral DNA from genomic viral RNA and the provirus is integrated into host genome by viral integrase [1]. The great majority of infections are caused by subtype-A (HTLV-1A) [3]. HTLV-1 is similar to HIV and can destroy the immune system by infecting CD4+ helper T-cells but instead of decreasing the number of CD4+ cells it causes proliferation of dysfunctional CD4+ cells. Unlike HIV infection; most patients infected with HTLV-1 remain asymptomatic throughout their lifetime [4].
Human T-Lymphocytic viruses belong to oncovirus family of retroviruses and can transform human lymphocytes so that they become self-sustaining in vitro, resulting in malignancies, inflammations, opportunistic infections, rheumatic and autoimmune conditions. The malignancies associated with HTLV-1 are well studied in Japan and include cutaneous T-cell Lymphoma and Adult T-cell Leukemia-Lymphoma which was first discovered in 1977 in Japan [1,2]. The inflammatory pathologies of HTLV-1 include uveitis and a demyelinating disease known as HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) whereas; opportunistic infections include pneumocystis jiroveci pneumonia (PJP) and strongyloides stercoralis. Furthermore, rheumatic and autoimmune conditions related to this virus include rheumatoid arthritis, Sjogren's syndrome, endemic polymyositis, bronchoalveolar pneumonitis, autoimmune thyroiditis and in some cases fibromyalgia. Chronic stimulation of the lymphocytes at the cytokine level may play a role in the development of the malignancies. The latent period for HTLV-1 infection varies geographically, ranging from 60 years in japan to less than 40 years in the Caribbean. Geographically, the highest prevalence of HTLV is seen in Japan (1-20%) followed by the Caribbean, Sub-Saharan African areas, Iran, Europe, central and south west of United States [4,7]. HTLV-1 transmission can occur through sexual contact, from mother to child via breastfeeding or from exposure to infected blood products and contaminated needles.
Clinically, ATLL can present with signs and symptoms of fever, fatigue, weight loss, malaise, lymphadenopathy, hypercalcemia along with tumor infiltrates of skin as rash. ATLL can also present in liver and spleen as hepatosplenomegaly (HSM), in lungs as infections and/or as lytic bone lesions. Most patients studied with HTLV-1 associated T-cell lymphomas have developed a syndrome of increased bone turnover and hypercalcemia at some time during the course of disease. 90% of patients with ATLL suffer from life threatening pulmonary complications including opportunistic lung infections such as PJP [4]. ATLL is generally categorized into four forms i.e. acute, chronic, smoldering and lymphomatous forms. Approximately 67% of the individuals can present with cutaneous lesions. The acute form comprises 55 to 75% of all cases of ATLL. In chronic ATLL variant skin lesions can be related to inflammatory processes, immunosuppression and/ or direct infiltration of the skin by neoplastic cells. Cutaneous lesions observed in chronic and smoldering variants of ATLL can be similar to those seen in patients suffering from mycosis fungoides. Monoclonal integration of provirus DNA into host cell's DNA is mandatory as a diagnostic proof of cutaneous manifestations of chronic and smoldering ATLL since the histopathological characteristics of both are similar to those seen for mycosis fungoides. Many types of skin lesions have been related to HTLV-1 infections such as macular, papular, nodular or tumoral lesions along with erythema, desquamation and/or erythroderma. The skin involvement in an otherwise asymptomatic individual carrying HTLV-1 can be an indicator of smoldering variant of ATLL [3].
Hypercalcemia occurs in about 70% of patients with HTLV- 1 induced ATLL, although smoldering and chronic subtypes of ATLL rarely develop hypercalcemia. Hypercalcemia seen in ATLL is more severe than in those with other hematologic malignancies. Several factors such as Interleukin-1 (1L-1), 1L-2, 1L-6, transforming growth factor-Beta (TGF-P), PTHrP, Tumor necrosis factor (TNF) and increased levels of 1-25 (OH)2 vitamin D have been implicated in ATLL-associated hypercalcemia. Among these factors PTHrP is considered to play an important role by stimulating osteoclasts (OCLs) resulting in increased bone resorption as ATLL cells constitutively express large amounts of PTHrP. In an experimental study, leukemic cells from a patient with ATLL were injected into immunodeficient mice which resulted in hypercalcemia and overexpressed PTHrP levels. However, PTHrP cannot directly induce differentiation of hematopoietic precursor cells (HPCs) to OCLs. Furthermore, in some patients hypercalcemia is not always associated with high levels of PTHrP, thus suggesting that another factor is involved in the pathogenesis of hypercalcemia.
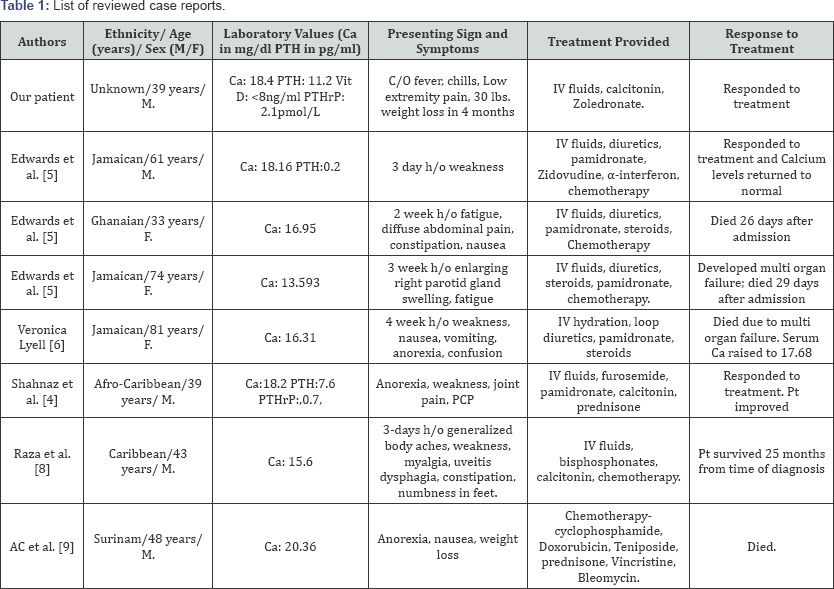
In a review of literature, Edwards et al. [5] presented a 61-year-old Jamaican man who was admitted with a 3-day history of generalized weakness (Table 1). He had high serum calcium level of 18.16mg/dl (reference range 8.9-10.1mg/dl), along with low PTH hormone level of 1.87pg/ml (10-65pg/ml). After aggressive treatment with intravenous fluids, diuretics and pamidronate, along with zidovudine, a-interferon and chemotherapy his calcium levels returned to normal. In another case, a 33 years old Ghanaian woman presented with 2-week history of fatigue, diffuse abdominal pain, constipation, and nausea with serum calcium levels were 16.95mg/dl. She was also treated with intravenous fluids, diuretics, pamidronate (bisphosphonate), steroids and chemotherapy; however, she died 26 days after admission. Same was the case of a 74-year- old Jamaican woman, who was admitted with a 3-week history of an enlarging right parotid gland swelling and fatigue. Her serum calcium level was 13.59mg/dl. She was treated with intravenous fluids, diuretics, steroids and intravenous pamidronate and chemotherapy. Ultimately, she developed multi-organ failure and passed away after 29 days of admission. In JMED Case Reports, Veronica Lyell et al. [6] presented a case of 81-year-old Jamaican woman, who lived in the UK for many years. She had a four week history of progressive malaise, weakness, nausea, vomiting, drowsiness, anorexia and confusion. Investigations revealed extreme hypercalcemia with corrected calcium level of 16.31mg/dl. The emergency management of hypercalcemia was intravenous hydration, loop diuretics and I.V pamidronate, which led to initial improvement in her serum calcium levels and consciousness. Her calcium levels, which had initially responded to therapy, abruptly increased to 17.68mg/dl. She was given high doses of steroids but she continued to deteriorate with high fever and died shortly afterwards due to multi-organ failure.
After a thorough analysis of comparative literature, consensus was made that ATLL is associated with worse prognosis when there is poor performance status, age over 40 years, elevated serum calcium levels, high levels of LDH, high WBCs and a large tumor size (Table 1). Out of the four subtypes discussed earlier, acute and lymphomatous subtypes have poor outcomes. Six months survival rate is almost 50% even when treated with a combination of chemotherapy, interferons and monoclonal antibodies against the interleukin-2 receptors. However, patients with chronic and smouldering subtypes have comparatively better prognosis and aggressive chemotherapy can be harmful rather than beneficial in these two subtypes [7]. In the review, hypercalcemia is a common finding in patients with HTLV-1 induced ATLL and the attempts to control calcium levels dominate the clinical course. Therefore, patients are initially treated with vigorous intravenous hydration, calcitonin, bisphosphonates and high dose steroids.
There is no licensed vaccination for HTLV-1 however, in the past two decades a large initiative has been taken to understand the biologic and pathogenic properties of the HTLV-1; which has ultimately led to the development of various experimental vaccinations and therapeutic strategies to combat HTLV-1 infection [8]. The treatment of ATLL is multi-agent cytotoxic chemotherapy and for HTLV a combination of Zidovudine and interferons can be used. Other potential treatment options such as prosultiamine (a vitamin B-1 derivative) showed a reduction in viral load and symptoms; azacytidine (an anti-metabolite) is credited for the cure of a patient in Greece. Cepharanthine, phosphonated carbocyclic 2'-oxa-3'aza nucleosides (PCOANs) and tenofovir alafenamide (TAF) are also promising treatment options [1,9,10].
Conclusion
The hypercalcemia in our patient occurred in the setting of near normal PTHrP and low 1-25 (OH)2 vitamin D levels, supporting the role for cytokines in the development of HTLV- 1 related hypercalcemia. Unfortunately, cytokines were not measured in our patient and this may represent a limitation of our paper. This case reminds us that patients with HTLV-1 infection may have different mechanisms inducing hypercalcemia. Cytokine mediated osteoclast resorption was probably the major mechanism for hypercalcemia for our patient. We believe the reason for hypercalcemia in our case was increased bone resorption as evidence by the positive response to intravenous Zoledronate.
References
- https://en.wikipedia.org/wiki/Human_T-lymphotropic_virus_1
- Poiesz BJ, Ruscetti FW, Reitz MS, Kalyanaraman VS, Gallo RC (1981) Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sezary T-cell leukaemia. Nature 294 (5838): 268-271.
- Gonsalves DU, Proietti FA, Ribas JG, Araujo MG, Pinheiro SR, et al. (2010) Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev 23(3): 577-589.
- Sabiha S, David R, Diana AV, Slavka K, Joanna T, et al. (2007) HTLV-1-Associated Adult T Cell Leukemia Lymphoma Presenting as Granulomatous Pneumocystis Jiroveci Pneumonia (PJP) and Hypercalcemia. Journal of General Internal Medicine 22(3): 420-423.
- Edwards CM, Edwards SJ, Bhumbra RP, Chowdhury TA (2003) severe refractory hypercalcemia in HTLV-1 infection. J R Soc Med 96(3): 126127.
- Lyell V, Khatamzas E, Allain T (2007) Severe hypercalcaemia and lymphoma in an HTLV-1 positive Jamaican woman: a case report. J Med Case Rep 1: 56.
- Gill PS, Harrington W, Kaplan MH, Ribeiro RC, Bennett JM, et al. (1995) Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med 332(26): 1744-1748.
- Raza S, Naik S, Kancharla VP, Tafera F, Kalavar MR (2010) Dual-Positive (CD4+/CD8+) Acute Adult T-Cell Leukemia/Lymphoma Associated with Complex Karyotype and Refractory Hypercalcemia: Case Report and Literature Review. Case Rep Oncol 3(3): 489-494.
- Tan AC, Janssens PM, Meijer JW, Mol JJ (2001) Hypercalcemia due to adult T-cell lymphoma in a man from Surinam. Ned Tijdschr Geneeskd 145(8): 370-374.
- Macchi B, Balestrieri E, Ascolani A, Hilburn S, Martin F, et al. (2011) Susceptibility of Primary HTLV-1 Isolates from Patients with HTLV-1- Associated Myelopathy to Reverse Transcriptase 1nhibitors. Viruses 3(12): 469-483.






























