Oocyte Quality and Female Infertility
Sharma A1, Gupta A1, Tiwari M1, Yadav PK1, Sahu K1, Prasad S1, Pandey AN1, Pandey AK2 and Chaube SK1*
1Cell Physiology Laboratory, Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi-221005, UP, India
2Department of Kayachikitsa, Faculty of Ayurveda, Institute of Medical Science, Banaras Hindu University, Varanasi-221005, UP, India.
Submission: December 21, 2017; Published: January 31, 2018
*Corresponding author: Shail K Chaube, Cell Physiology Laboratory, Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi-221005, India, Tel: 91-542-26702516, Fax: 91-542-2368174; Email: shailchaubey@gmail.com; shailchaube@bhu.ac.in
How to cite this article: Sharma A, Gupta A, Tiwari M, Sahu K, Prasad S Et Al. Oocyte Quality and Female Infertility. Glob J Reprod Med. 2018; 3(2): 555607. DOI: 10.19080/GJORM.2018.03.555607
Abstract
Female infertility is one of the major reproductive health issue affecting majority of women worldwide. Several factors including environmental, hormonal and physical may affect the physiology of ovary to release quality grade oocyte required for fertilization and early embryonic development. The quality of oocyte is dependent on several factors within the follicular microenvironment and even after ovulation. One of the major factors that affect oocyte quality is the induction of apoptosis. Apoptosis plays a major role to eliminate majority of germ cells from the cohort of ovary during various stages of folliculogenesis. Few numbers of oocytes are selectively recruited to get ovulated during entire reproductive life span in female. Prior to ovulation, these oocytes achieve meiotic competency that may last for several months in rodents to several years in human. Inability to achieve meiotic competency within the follicular microenvironment and spontaneous egg activation (SEA) immediately after ovulation may deteriorate oocyte quality. Thus, induction of apoptosis or meiotic arrest at Metaphase-I stage (M-I) or SEA could reduce female fertility and may cause infertility.
Keywords: Apoptosis; Oocyte competency; Spontaneous egg activation; Ovary; Female infertility
Abbreviations: SEA: Spontaneous Egg Activation; M-I: Metaphase-I; M-II: Metaphase-II; M-III: Metaphase-III; PB-I: First Polar Body; PB-II: Second Polar Body; ROS: Reactive Oxygen Species
Introduction
Infertility is a one of the major reproductive health problems that has affected almost 10% of young age group worldwide. The infertility rate remains unchanged over past two decades besides having significant advancement in reproductive health sector [1]. This could be due to environmental, stress, lifestyle factor, hormonal and pathophysiological factors [2]. These factors directly or indirectly affect the physiology of ovary that is responsible for the generation of competent oocytes for fertilization and early embryonic development [3]. The increase of stress hormone induces granulosa cell apoptosis responsible for synthesis of estradiol-17β. Estradiol depletion at the level of ovary affects follicular growth and development [2]. Amelioration in follicular growth and development induces follicular atresia [4]. The increased stress causes oxidative stress and reactive oxygen species (ROS) at the level of ovary trigger germ cell depletion via apoptosis [5]. Several factors and pathways facilitate germ cell depletion at all the stages of oogenesis in mammals [6]. The large number of germ cells is eliminated from the cohort of ovary just before the attainment of puberty [4]. At puberty, less than 1% of germ cells remains in the ovary that are subjected to selective recruitment process during entire reproductive life span [7].
The selective recruitment of oocytes during puberty in response to pituitary gonadotrophin surge induces meiotic resumption from diplotene arrest in follicular oocytes by increasing the level of cyclic nucleotides as well as Mos level in granulosa cells of follicular oocytes [8]. These cyclic nucleotides and MOS/MEK/MAPK signalling pathways disrupt the gap junctions between granulosa cells and oocytes resulting in a transient decrease of oocyte adenosine 3',5'-cyclic monophosphate (cAMP) required to maintain diplotene arrest in follicular microenvironment [9]. A transient decrease of oocyte cAMP activates mitogen-activated protein kinase (MAPK) as well as cyclin dependent kinasel (Cdkl), a catalytic unit of maturation promoting factor (MPF). Further, decrease of cAMP destabilizes MPF [10]. The MPF destabilization causes meiotic resumption from diplotene arrest and oocyte progresses towards to metaphase-I stage (M-I) [11]. The M-I arrest may last for very short period of time in vivo and oocyte progresses to reach metaphase-II stage (M-II) by extruding first polar body (PB-I) at the time of ovulation [12]. However, removal of oocyte from follicular microenvironment and their culture in vitro results in spontaneous resumption of meiosis but they are unable to progress beyond M-I under in vitro culture conditions [13].
These oocytes are unfit for fertilization as they contain diploidset of chromosomes and do not posses PB-I. Further, growing body of evidences suggest that the oocytes after ovulation do not wait for fertilizing spermatozoa and quickly undergo meiotic exit from M-II arrest so called spontaneous activation in several mammalian species [14,15]. The spontaneous activation is possibly due to premature release of calcium (Ca++) from internal stores and increase of cytosolic free calcium. A moderate increase of cytosolic free calcium triggers downstream pathway to destabilize MPF [16]. MPF destabilization results spontaneous activation by initiating the extrusion of second polar body (PB-II). These oocytes are of poor quality and their use limits reproductive outcome and may trigger infertility problems [17].
Apoptosis and oocyte quality
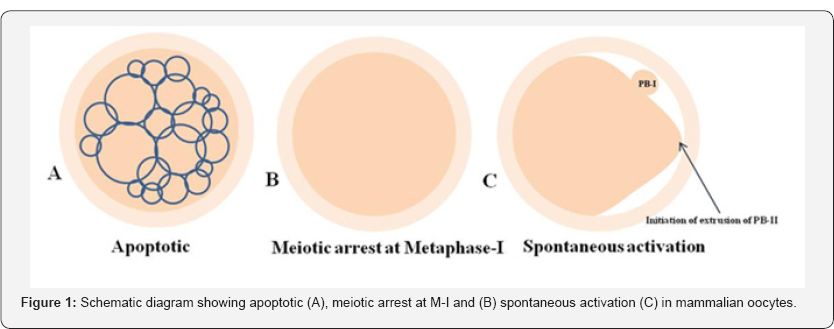
Apoptosis plays a major role in follicular atresia and eliminates majority of defective as well as surplus germ cells from the cohort of ovary [18,19]. By this way, ovary keeps only few numbers of germ cells (less than 1%) for selective recruitment during entire reproductive lifespan. As the aging occurs, decline of number of follicles below threshold level may cause infertility [20,21]. Studies suggest that the good quality of oocyte is ovulated first and as the maternal aging occurs, poor quality oocytes are remained in the ovary. These oocytes are more fragile and susceptible towards apoptosis that reduces reproductive outcome (Figure 1) [22-24]. Women are more frequently exposed to various kinds of stress during their reproductive period [25]. The psychological stress, lifestyle changes and various other factors stimulate the release of stress hormone and reactive oxygen species (ROS) [2]. The increased level of stress hormone and ROS induce apoptosis not only in granulosa cells but also in follicular oocytes [5,26]. There are several players and both as death receptors as well as mitochondria-mediated pathways involved in oocyte apoptosis within the follicle of the ovary [27,28]. Indeed, apoptosis plays a major role in determining the quality of follicular oocytes that directly affects reproductive outcome of a female and induces infertility [4].
Meiotic maturation arrest and oocyte quality
Meiotic maturation is required for the follicular oocytes to achieve developmental competency [29]. The achievement of meiotic competency starts with the resumption from diplotene arrest in follicular oocytes and ends with extrusion of PB-I [16]. Any defect during the achievement of meiotic competency does not allow the follicular oocyte to progress meiosis [30]. These compromised oocytes are arrested at M-I stage and do not progress to extrude PB-I [12,13,31]. Further, M-II arrested oocytes even after insemination do not get activated [32]. These oocytes are of poor quality due to meiotic maturation arrest either at M-I stage or at M-II stage under in vitro culture conditions (Figure 1B) [3,33]. The meiotic maturation failure could be possibly due to maintenance of high level of stabilized MPF. The high level of stabilized MPF is required for the maintenance of meiotic arrest [34,35]. The meiotic maturation arrest may cause infertility in human [3].
Spontaneous activation and oocyte quality
The oocyte after ovulation are generally arrested at M-II stage and posses PB-I in most of the mammalian species [3538]. Growing body evidences suggest that oocyte do not wait for fertilizing spermatozoa and quickly undergo spontaneous exit from M-II arrest in several mammalian species including human [39-42]. The initiation of extrusion of PB-II starts but never gets completely extrude (Figure 1C). Oocytes are further arrested at Metaphase-III (M-III) like stage [43].The SEA could be due to abortive increase of cytosolic free calcium and activation of downstream pathway to destabilize MPF [37,38,44]. A moderate increase of cytosolic free Ca++ is good enough to trigger SEA but not sufficient to induce full activation process [37,44]. These oocytes are not fit for fertilization since the chromosomes are scattered throughout the cytoplasm. A large amount of cytoplasm goes towards the side of polar body formation but PB-II never completely extruded [11]. These oocytes are of poor quality and cannot be used for any assisted reproductive technology (ART) program including somatic cell nuclear transfer program (SCNT) during animal cloning [36,11].
Conclusion
Good quality of oocytes is the right choice for fertilization and early embryonic development. Deterioration in oocyte quality may occur due to the onset of apoptosis in the follicular oocytes. Majority of oocytes are eliminated from ovary via apoptosis during follicular atresia. Only few oocytes remain in the ovary that are selectively recruited for ovulation during entire reproductive life of a female. Prevention of MPF destabilization may cause meiotic maturation arrest in follicular oocytes. After ovulation, oocyte quality undergoes Ca++ mediated MPF destabilization that causes SEA in several mammalian species including human. Thus, apoptosis in oocytes, meiotic maturation arrest and SEA may deteriorate oocyte quality after ovulation. Poor quality oocyte directly impacts the reproductive outcome and causes female infertility.
References
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA (2012) National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 9(12): e1001356.
- Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK (2016) Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci 23: 36.
- Mrazek M, Fulka J (2003) Failure of oocyte maturation: Possible mechanisms for oocyte maturation arrest. Hum Reprod 18(11): 22492252.
- Tiwari M, Prasad S, Tripathi A, Pandey AN, Ali I, et al. (2015) Apoptosis in mammalian oocytes: A review. Apoptosis 20(8): 1019-1025.
- Chaube SK, Prasad PV, Thakur SC, Shrivastav TG (2005) Hydrogen peroxidemodulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis 10(4): 863-874.
- Barrett SL, Albertini DF (2010) Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence. J Assist Reprod Genet 27(1): 29-39.
- Tilly JL (2001) Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol 2(11): 838-848.
- Wassarman PM, Albertini DF (1994) The mammalian ovum. In: Knobil E, Neill JD (Eds.) The physiology of reproduction. Raven-Press, New York, USA, pp. 79-122.
- Gupta A Tiwari M, Prasad S, Chaube SK (2017) Role of cyclic nucleotide phosphodiesterase during meiotic resumption from diplotene arrest in mammalian oocytes. J Cell Biochem 118(3): 446-452.
- Tiwari M, Gupta A, Sharma A, Prasad S, Pandey AN, et al. (2017) Role of Mitogen Activated Protein Kinase and Maturation Promoting Factor During the Achievement of Meiotic Competency in Mammalian oocytes. J Cell Biochem 119(1): 123-129.
- Chaube SK, Prasad S, Tiwari M, Gupta A (2016) Rat: an interesting model to study oocyte meiosis in mammals. Research and Reviews: Journal of Zoological Sciences 4(3): 25-27.
- Tripathi A, Kumar KV, Chaube SK (2010) Meiotic cell cycle arrest in mammalian oocytes. J Cell Physiol 223(3): 592-600.
- Tiwari M, Tripathi A, Chaube SK (2017) Presence of encircling granulosa cells protects against oxidative stress-induced apoptosis in rat eggs cultured in vitro. Apoptosis 22(1): 98-107.
- Tiwari M, Prasad S, Shrivastav TG, Chaube SK (2017) Calcium signaling during meiotic cell cycle regulation and apoptosis in mammalian oocytes. J Cell Physiol 232(5): 976-981.
- Prasad S, Tiwari M, Koch B, Chaube SK (2015) Morphological, cellular and molecular changes during postovulatory aging in mammals. J Biomed Sci 22: 36.
- Tiwari M, Chaube SK (2016) Moderate increase of reactive oxygen species triggers spontaneous meiotic resumption in rat follicular oocytes. J ObstetGynecol Res 42(5): 536-546.
- Miao YL, Kikuchi K, Sun QY, Schatten H (2009) Oocyte aging: Cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod update 15(5): 573-585.
- Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4): 239-257.
- Myers M, Morgan FH, Liew SH, Zerafa N, Gamage TU, et al. (2014) PUMA regulates germ cell loss and primordial follicle endowment in mice. Reproduction 148(2): 211-219.
- Matsuda F, Inoue N, Manabe N, Ohkura S (2012) Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev 58(1): 44-50.
- Liew SH, Nguyen QN, Strasser A, Findlay JK, Hutt KJ (2017) The ovarian reserve is depleted during puberty in a hormonally driven process dependent on the proapoptoticprotein BMF. Cell Death Dis 8(8): e2971.
- Wu J, Zhang L, Wang X (2000) Maturation and apoptosis of human oocytes in vitro are age-related. FertilSteril 74(6): 1137-1141.
- Santonocito M, Guglielmino MR, Vento M, Ragusa M, Barbagalla D, et al. (2013) The apoptotic transcriptome of the human MII oocyte: characterization and age-related changes. Apoptosis 18(2): 201-211.
- Tsutsumi M, Fujiwara R, Nishizawa H, Ito M, Kogo H, et al. (2014) Age- related decrease of meiotic cohesins in human oocytes. PLoS One 9(5): e96710.
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, et al. (2008) A new model of reproductive aging: The decline in ovarian nongrowing follicle number from birth to menopause. Hum Reprod 23(3): 699-708.
- Chaube SK, Prasad PV, Thakur SC, Shrivastav TG (2005) Estradiol protects clomiphene citrate-induced apoptosis in ovarian follicular cells andovulated cumulus-oocyte complexes. Fertil Steril 84(2): 11631172.
- Aitken RJ, Findlay JK, Hutt KJ, Kerr JB (2011) Apoptosis in the germ line. Reproduction 141(2): 139-150.
- Hutt KJ (2015) The role of BH3-only proteins in apoptosis within the ovary. Reproduction 149(2): R81-R89.
- Chaube SK (2001) Role of meiotic maturation regulatory factors in the developmental competence of mammalian oocytes. Health and Population 24(4): 218-231.
- Mehlmann LM (2005) Stops and starts in mammalian oocytes: Recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 130(6): 791-799.
- Kubiak JZ, CiemerychMA, Hupalowska A, Sikora-Polaczek M, Polanski Z (2008) On the transition from the meiotic cell cycle during early mouse development. Int J Dev Biol 52(2-3): 201-217.
- Pandey AN, Tripathi A, Premkumar KV, Shrivastav TG, Chaube SK (2010) Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J Cell Biochem 111(3): 521528.
- Pandey AN, Chaube SK (2014) A moderate increase of hydrogen peroxide level is beneficialfor spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured invitro. Biores Open Access 3(4): 183-191.
- Tiwari M, Chaube SK (2017) Maturation promoting factor destabilization mediates human chorionic gonadotropin induced meiotic resumption in rat oocytes. Dev Growth Differ 59(7): 603-614.
- Prasad S, Tiwari M, Tripathi A, Pandey AN, Chaube SK (2015) Changes in signal molecules and maturation promoting factor levels associate with spontaneous resumption of meiosis in rat oocytes. Cell Biol Int 39(6): 759-769.
- Chebotareva T, Taylor J, Mullins JJ, Wilmut I (2011) Rat eggs cannot wait: Spontaneous exit from meiotic metaphase II arrest. Mol Reprod Dev 78(10-11): 795-807.
- Premkumar KV, ChaubeSK (2013) An insufficient increase of cytosolic free calcium level results postovulatory aging-induced abortive spontaneous egg activation in rat. J Assist Reprod Genet 30(1): 117123.
- Prasad S, Koch B, Chaube SK (2016) Maturation promoting factor destabilization facilitates postovulatory aging-mediated abortive spontaneous egg activation in rat. Dev Growth Differ 58(3): 293-302.
- Kim NH, Moon SJ, Prather RS, Day BN (1996) Cytoskeletal alterationin aged porcine oocytes and parthenogenesis. Mol Reprod Dev 43(4): 513-538.
- Kim NH, Chung HM, Cha KY, Chung KS (1998) Microtubule and microfilament organization in maturing human oocytes. HumReprod 13(8): 2217-2222.
- LiG P, Liu Y, Bunch TD, White KL, Aston KI (2005) Asymmetric division of spindle microtubules and microfilaments during bovine meiosis from metaphase I to metaphase III. Mol Reprod Dev 71(2): 220-226.
- Combelles CM, Albertini DF, Racowsky C (2003) Distinct microtubule and chromatin characteristics of human oocytes after failed invivo and invitro meiotic maturation. Hum Reprod 18(10): 2124-2130.
- Ross PJ, Yabuuchi A, Cibelli JB (2006) Oocyte spontaneous activation in different rat strains. Cloning Stem Cells 8(4): 275-82.
- Premkumar KV, Chaube SK (2014) RyR channel-mediated increase of cytosolic free calcium level signals cyclin B1 degradation during abortive spontaneous egg activation in rat. In Vitro Cell Dev Biol Anim 50(7): 640-647.






























