Cultivating a Support Network: Granulosa Cells and the Needed Knowledge on their Role in Oocyte Maturation
Dinorah Hernández-Melchor, Bruno López-Bayghen and Esther López-Bayghen*
Department of Toxicology, Cinvestav-IPN, Mexico
Submission: April 12,2017; Published: May 05, 2017
*Corresponding author: Esther López-Bayghen, Department of Toxicology, Cinvestav-IPN, Mexico, Tel: +52-55-5747-3800; Fax: +52-55-5747-3395; Email: ebayghen@cinvestav.mx
How to cite this article: Hernandez D, López-Bayghen B, López-Bayghen E. Cultivating a Support Network: Granulosa Cells and the Needed Knowledge on their Role in Oocyte Maturation. Glob J Reprod Med. 2017; 1(2): 555556. DOI: 10.19080/GJORM.2017.01.555556
Abstract
Maintaining and restoring full developmental competence of oocytes after a process of cryopreservation to preserve fertility is a growing concern in the field of assisted reproductive technologies (ART). Unfortunately, attempts so far have been characterized by low quality and a poor yield of live births. A promising avenue of research is to replicate the microenvironment of the follicles to achieve careful cryopreservation and successful in vitro maturation. A key step for this is to understands the interactions between somatic granulosa cells and the oocyte that are required for maturation and developmental competence. In the present work we present a succinct review of these molecular interactions, including endocrine and paracrine signaling, gene regulation and cell communication. We focus primarily on the role of both types of granulosa cells: mural granulosa cells, which perform endocrine functions, including hormone production and cumulus cells, which provide metabolic support and establish bi-directional communication with the oocyte through various pathways, including direct contact. The coordinate development of both granulosa cell types is necessary to fulfill oocyte requirements. Being able to reproduce those conditions in vitro could lead to successful oocyte maturation and positive fertilization outcomes.
Keywords: Granulosa cells; Cumulus; Mural cells; Cumula-oocyte complex; Oocyte maturation; Granulosa cell culture
Introduction
Maintaining and restore the full developmental competence of either mature or immature oocytes after cryopreservation processes to preserve women fertility is a goal in assisted reproductive technologies (ART). This goal arises from the need of some patients to preserve their oocytes before arriving to a reproductively mature age, such as patients who need to undergo chemotherapy. Although in vitro fertilization (IVF) can be performed to store embryos for future use with cumulative pregnancy rates >60% [1], this may not be an option for single women or patients without time to complete ovarian stimulation prior to cancer therapy. For those patients, oocyte cryopreservation can be considered instead [1-4]. Data from 21 peer-reviewed journals show that mean survival rate of frozen- thawed mature oocytes is 47%, while mean fertilization rate is 52.5% with a mean pregnancy rate for thawed oocytes of only 1.52% [1]. It has been suggested that immature oocytes may be more resistant to cryodamage due to lower cell volume and lack of metaphase spindle. Even though high rates of nuclear maturation have been reported with cryopreserved immature oocytes instead [1,3], the developmental capacity has been generally low [1].
The processes involved in oocyte maturation includes resumption of meiosis form prophase I, when the oocyte is in Germinal Vesicle stage (GV), to the extrusion of the first polar body, in Metaphase II (MII); the expansion of somatic cells surrounding the oocyte and the maturation of the cytoplasm to support fertilization and early embryonic development [5]. Whether the maturation happens in vivo or in vitro, the environment to which the oocyte and its surrounding cells are exposed, affects the developmental competence of the oocyte and subsequent embryonic development [5]. Oocytes within the ovarian follicle are surrounded by mural and cumulus granulosa cells, which perform both endocrine and developmental functions, respectively. The coordinate function of both granulosa cell types is necessary to fulfill oocyte requirements. A critical difference between cryopreserving MII or GV stage oocytes centers on the importance of the intercellular contacts between germinal and somatic cells. MII-stage oocytes do not rely on support from surrounding somatic cells, while cryopreservation of GV-stage oocytes requires maintenance of their communication with viable somatic cells to preserve a mutual interaction [4]. In both cases, being able to reproduce the conditions in vitro by recreating the follicle microenvironment using cultured granulosa cells may be necessary for successful oocyte maturation, either before or after freezing.
Mural granulosa cells line the follicle wall and form a stratified epithelium with the basal lamina [6]. This cell type express luteinizing hormone receptors (LHCGR), its expression is induced after human chorionic gonadotropin stimulation. LH signaling induces the expression of progesterone receptor and other ovulation-related genes, including the protease ADAMTS-1 and its substrate Versican, a large chondroitin-sulphate- substituted aggregating proteoglycan of the lectican family. ADAMTS-1 and Versican are secreted and selectively relocated to the Extracellular Matrix (ECM) during cumulus expansion [7]. The principal physiological functions of MGC are the production of estradiol and progesterone [6-8]: CYP11A1 converts cholesterol to pregnenolone, next HSD3B1 converts it into progesterone. On the other hand, CYP19A1 uses androstenedione and testosterone as precursors to produce estrone and estradiol, also HSD17B converts estrone to estradiol [9-11].
Cumulus cells are highly specialized cells that establish direct contact with the oocyte to promote its growth and development though bi-directional interactions [6-8]. The cumulus cells have transzonal cytoplasmic processes, which penetrate through the zona pellucida and abut the oocyte membrane, forming the cumulus-oocyte complex (COC) [8]. The oocyte depends on cumulus cells for metabolic support. Those cells provide the oocyte the nutrients and regulatory signals to facilitate the progression of maturation, while oocyte secreted factors acts as signaling molecules to allow cumulus cell differentiation from mural granulosa cells [5,7,8]. Cumulus cells exhibit a high capacity to metabolize glucose to use it both as an energy source or to produce HA for cumulus expansion [5,12,13]. Cumulus cells glucose uptake is mediated by two separate glucose transporters: SLC2A1 and SLC2A4 [5]. Once inside CCs, glucose is directed to one of four metabolic pathways. A high proportion of this glucose enters the glycolytic pathway and by the action of enzymes such as phosphofructokinase is metabolized to produce ATP, pyruvate and lactate, metabolites that can be easily used as energy sources for both cumulus cells and the oocyte [5,12]. The remaining fraction is metabolized by the hexosamine biosynthesis pathway, where glucose-6-phosphatetofructose-6-phosphate is converted to glucosamine-6-phosphate by glucosamine fructose- 6-phosphate transaminase (GFPT), and the end product of the pathway is UDP-N-acetyl glucosamine [5]. UDP-N-acetyl glucosamine is then used for cumulus matrix expansion as it is converted to hyaluronic acid (HA) by hyaluronic acid synthase 2 (HAS2) or to protein posttranslational modification via ᵦ-O- linked glycosylation by the action of O-linked glycosylation transferase [5,13]. Cumulus cells also express TGF-beta type I receptor (ALK5) and Bone Morphogenetic Protein Receptor 2 (BMPR2). These receptors allow for the activation of pathways mediated by TGFp related proteins secreted by the oocyte like the growth differentiation factor 9 (GDF9) and bone morphogenetic protein (BMP15), which are important mediators for the follicle growth, cumulus cells differentiation and cumulus expansion [7,8,14,15].
Cumulus expansion during oocyte maturation involves the synthesis of ECM by cumulus cells in response to the LH surge in vivo [5,7,8,13] and epidermal growth factor (EGF) [5,13] or follicle stimulation hormone (FSH) stimulation in vitro [5]. After LH stimulation, cumulus cells express the HA surface receptors CD44 and the hyaluronan-mediated motility receptor (RHAMM), which bind to HA and allow the formation of the ECM matrix between cumulus cells. Versican interacts with HA providing strength and elasticity while being specifically cleaved by ADAMTS-1 which is selectively accumulated in the ECM, to allow for normal matrix function. Two other proteins expressed by CC, Pentraxin 3 (PTX3) and TNF-alpha induced protein 6 (TNFAIP6) interact to assemble and stabilize the matrix [7,8,15,16]. Active components of the ECM are synthesized directly by cumulus cells under the instruction of oocyte-derived factors and endocrine factors secreted by mural granulosa cells or enter the follicle through blood plasma [14]. The coordinate development of both granulosa cell types is necessary to fulfill the oocyte's requirements.
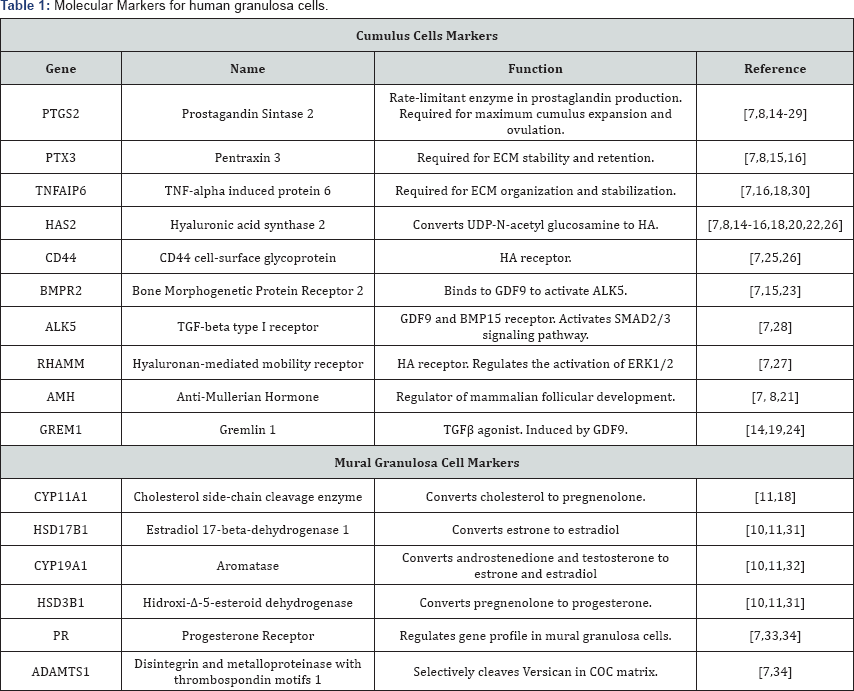
Given that mural granulosa cells exhibit hormone responsiveness and gene expression profiles distinct from that of cumulus cells, it is important to establish specific molecular markers of each cell type to be able to characterize them when in culture. For mural granulosa cells hormone receptors, ovulatory- related proteins and steroidogenic enzymes are potential markers. In cumulus cells receptors for oocyte-derived factors, glycolytic and hexosamine biosynthesis pathway (HBP) related enzymes, and proteins necessary for cumulus expansion are ideal markers. Additionally, several genes have been identified to be expressed specifically in each granulosa cell type and to play crucial roles, potentially could be used as well. In Table 1 we summarized the proposed markers that can be used to characterize both mural and cumulus granulosa cells. All the molecules have been reported to be detected in human granulosa cells at least once.
Conclusion
Establishing in vitro models for both cumulus and granulosa cells is an essential step in order to provide working material for oocyte maturation. Furthermore, well characterized models to study these cells will provide research tools to understand the complex interplay between these cells and the oocyte. This may open even more leads into understanding how external factors, such as endocrine disruptors and environmental pollutants can affect oocyte quality. Without a doubt, expanding our research on granulosa cells will become fundamental in the field of reproductive biology in the coming years.
References
- Sonmezer M, Oktay K (2004) Fertility preservation in female patients. Hum reprod update 10(3): 251-266.
- Wang X, Gook DA, Walters KA, Anazodo A, Ledger WL, et al. (2016) Improving fertility preservation for girls and women by coupling oocyte in vitro maturation with existing strategies. Women's health 12(3): 275-278.
- Seli E, Tangir J (2005) Fertility preservation options for female patients with malignancies. Curr Opin Obstet Gynecol 17(3): 299-308.
- Brambillasca F, Guglielmo MC, Coticchio G, Mignini Renzini M, Dal Canto M, et al. (2013) The current challenges to efficient immature oocyte cryopreservation. Journal of assisted reproduction and genetics 30(12): 1531-1539.
- Sutton-McDowall ML, Gilchrist RB, Thompson JG (2010) The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 139(4): 685-695.
- Gilchrist RB, Ritter LJ, Armstrong DT (2004) Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci 82-83: 431-446.
- Russell DL, Robker RL (2007) Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum reprod update 13(3): 289-312.
- Diaz FJ, Wigglesworth K, Eppig JJ (2007) Oocytes determine cumulus cell lineage in mouse ovarian follicles. J cell sci 120(Pt 8): 1330-1340.
- Craig ZR, Wang W, Flaws JA (2011) Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction 142(5): 633-646.
- Belani M, Purohit N, Pillai P, Gupta S, Gupta S (2014) Modulation of steroidogenic pathway in rat granulosa cells with subclinical Cd exposure and insulin resistance: an impact on female fertility. Bio Med research international 2014: 460251.
- Hannon PR, Flaws JA (2015) The effects of phthalates on the ovary. Front Endocrinol (Lausanne) 6: 8.
- Sutton-McDowall ML, Gilchrist RB, Thompson JG (2004) Cumulus expansion and glucose utilisation by bovine cumulus-oocyte complexes during in vitro maturation: the influence of glucosamine and follicle- stimulating hormone. Reproduction 128(3): 313-319.
- Richani D, Sutton-McDowall ML, Frank LA, Gilchrist RB, Thompson JG (2014) Effect of Epidermal Growth Factor-Like Peptides on the Metabolism of In Vitro-Matured Mouse Oocytes and Cumulus Cells 1. Bio reprod 90(3): 49.
- McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, et al. (2004) Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Human reproduction 19(12): 2869-2874.
- Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, et al. (2013) Growth differentiation factor 9: bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci 110(8): E776-785.
- Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ (2006) The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol 299(1): 91-104.
- Marei WF, Abayasekara DR, Wathes DC, Fouladi-Nashta AA (2014) Role of PTGS2-generated PGE2 during gonadotrophin-induced bovine oocyte maturation and cumulus cell expansion. Reprodu biomed online 28(3): 388-400.
- Prochazka R, Petlach M, Nagyova E, Nemcova L (2011) Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: comparison with gonadotropins. Reproduction 141(4): 425-435.
- Liu Q, Li Y, Feng Y, Liu C, Ma J, et al. (2016) Single-cell analysis of differences in transcriptomic profiles of oocytes and cumulus cells at GV, MI, MII stages from PCOS patients. Sci Rep 6: 39638.
- Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JN, et al. (2004) Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Developmental biology 276(1): 64-73.
- Kedem A, Yung Y, Yerushalmi GM, Haas J, Maman E, et al. (2014) Anti Mullerian Hormone (AMH) level and expression in mural and cumulus cells in relation to age. J ovarian Res 7: 113.
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Armstrong DT, et al. (2005) Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology 146(6): 2798-2806.
- Reader KL, Mottershead DG, Martin GA, Gilchrist RB, Heath DA, et al. (2016) Signalling pathways involved in the synergistic effects of human growth differentiation factor 9 and bone morphogenetic protein 15. Reprod Fertil Dev 28(4): 491-498.
- Jindal S, Greenseid K, Berger D, Santoro N, Pal L (2012) Impaired gremlin 1 (GREM1) expression in cumulus cells in young women with diminished ovarian reserve (DOR). J Assist Reprod Genet 29(2): 159-162.
- Ohta N, Saito H, Kuzumaki T, Takahashi T, Ito MM, et al. (1999) Expression of CD44 in human cumulus and mural granulosa cells of individual patients in in-vitro fertilization programmes. Mol Hum Reprod 5(1): 22-28.
- Chavoshinejad R, Marei WF, Hartshorne GM, Fouladi-Nashta AA (2016) Localisation and endocrine control of hyaluronan synthase (HAS) 2, HAS3 and CD44 expression in sheep granulosa cells. Reprod Fertil Dev 28(6): 765-775.
- Li H, Moll J, Winkler A, Frappart L, Brunet S, et al. (2015) RHAMM deficiency disrupts folliculogenesis resulting in female hypofertility. Biol Open 4(4): 562-571.
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, et al. (2007) Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod 76(5): 848857.
- Purcell SH, Chi MM, Lanzendorf S, Moley KH (2012) Insulin-stimulated glucose uptake occurs in specialized cells within the cumulus oocyte complex. Endocrinology 153(5): 2444-2454.
- Assou S, Haouzi D, Dechaud H, Gala A, Ferrieres A, et al. (2013) Comparative gene expression profiling in human cumulus cells according to ovarian gonadotropin treatments. Biomed Res Int 2013: 354582.
- Paradis F, Moore HS, Pasternak JA, Novak S, Dyck MK, et al. (2010) Pig preovulatory oocytes modulate cumulus cell protein and gene expression in vitro. Mol Cell Endocrinol 320(1-2): 87-96.
- Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, et al. (2006) Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol 20(6): 1300-1321.
- Lei ZM, Mishra S, Zou W, Xu B, Foltz M, et al. (2001) Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol endocrinol 15(1): 184-200.
- Guzman L, Adriaenssens T, Ortega-Hrepich C, Albuz FK, Mateizel I, et al. (2013) Human antral follicles <6 mm: a comparison between in vivo maturation and in vitro maturation in non-hCG primed cycles using cumulus cell gene expression. Mol Hum Reprod 19(1): 7-16.






























