Increased Expression of Interleukin (IL)-17, -22, -23, and -26 mRNAs in Patients with Otitis Media
Su Young Jung1, Myung Gu Kim2, Jeon Gang Doo1, Sung Su Kim3, Young Il Kim4, Sang Hoon Kim1 and Seung Geun Yeo14*
1Department of Otorhinolaryngology-Head and Neck Surgery, Kyung Hee University, Korea
2Department of Otorhinolaryngology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Republic of Korea
3Department of Biochemistry and Molecular Biology, Medical Science and Engineering Research Center for Bioreaction to Reactive Oxygen Species, BK-21, School of Medicine, Kyung Hee University, Korea
4Medical Science Research Institute, Kyung Hee University Medical Center, Kyung Hee University, Republic of Korea
Submission: April 11, 2018; Published: May 02, 2018
*Corresponding author: Seung Geun Yeo, Department of Otorhinolaryngology - H & N Surgery, Graduate School, Kyung Hee University. #1 Hoegi- dong, Dongdaemun-gu, Seoul 02447, Korea, Tel: 82-2-958-8474, Fax: 82-2-958-8470; Email: yeo2park@gmail.com
How to cite this article: Su Young J, Myung Gu K, Sung Su K, Young Il K,Seung G Y,et.al. Increased Expression of Interleukin (IL)-17, -22, -23, and -26 mRNAs in Patients with Otitis Media. Glob J Oto, 2018; 15(3): 555915. DOI: 10.19080/GJO.2018.15.555915.
Abstract
Objective: This study therefore evaluated the expression of interleukin (IL)-17, IL-22, IL-23, and IL-26 mRNAs in patients with OM with effusion (OME), chronic OM (COM), and cholesteatomatous OM (CholeOM) and the relationships between their expression and the absence of bacteria and hearing loss.
Methods: 87 Patients diagnosed with OME, COM, and CholeOM from 2013 to 2016 were enrolled. Effusion fluid, granulation tissue, and cholesteatoma samples were obtained during surgery. Levels of IL-17, IL-22, IL-23, and IL-26 mRNAs were assessed by real-time PCR and compared the results according to types of OM, result of bacterial culture, and type of hearing loss.
Results: IL-17, IL-22, IL-23, and IL-26 mRNAs were expressed in all three groups of patients with OM, with all being significantly higher in patients with OME than in patients with COM and CholeOM (p <0.05), but not differing significantly in the latter two groups. The increased levels of all four mRNAs in patients with OME were independent of the presence or absence of bacteria and of the type of hearing loss (p <0.05).
Conclusion: IL-17, IL-22, IL-23, and IL-26 are involved in the pathophysiology of OM and are significantly higher in patients with OME than in patients with COM and CholeOM.
Keywords: Otitis media; Interleukins; Otitis media with effusion (OME); Chronic otitis media (COM); Cholesteatoumaotus Otitis Media; (choleOM)
Introduction
Otitis media (OM) is a common disease among children in both developing and developed countries [1]. About 80% of children under age 3 years experience OM at least once and about 40% of children under age 7 years experience more than six episodes of OM [2]. OM in children is caused by complex interactions among multiple factors, including Eustachian tube dysfunction, allergy, bacterial and viral infections, dysfunction of cilia within the middle ear and mucous membrane of the Eustachian tube, exposure to smoke, esophageal reflux, and placement in daycare facilities [3,4]. Host immune responses to viral and bacterial infections were reported to be closely associated with complete recovery from OM, as well as with the persistence and recurrence of this disease [1,4].
Chronic OM (COM) is also caused by multiple factors, but the mechanisms by which an acute infection within the middle ear and mastoid cavity becomes chronic have not been determined. Osteoclasia, which is common in patients with cholesteatomatous OM (CholeOM), is regarded as being due to the pressure created by the cholesteatoma itself. Enzymes secreted by granulation tissue that develops during immune responses, including collagenase, acid phosphatase, and acid protease, as well as cytokines secreted by inflammatory cells, have been reported to influence the occurrence of osteoclasia [5,6]. Innate and adaptive immune responses within the middle ear cavity of patients with various types of OM have therefore been evaluated, including assessments of cytokine secretion [7-9].
Although many studies have focused on the roles of cytokines in patients with otitis media with effusion (OME), COM, and CholeOM, cytokine expression in these three groups has, to our knowledge, not been compared. Moreover, little is known about the roles of interleukin (IL)-17, -22, -23, and -26 in OM. This study therefore evaluated the expression levels of those four cytokines in groups of patients with OME, COM, and CholeOM, and their association between the presence or absence of bacteria and with various types of hearing.
Materials and Methods
Subjects study design
Samples were obtained from 87 patients with OM, including 39 with chronic OME, 24 with COM, and 24 with CholeOM, who visited the Department of Otorhinolaryngology-Head and Neck Surgery at single university hospital between July 2013 and June 2017 to undergo ventilation tube insertion and/or tympanomastoidectomy. OME was diagnosed by the presence on otoscopic examination of an amber-colored tympanic membrane and by the presence on impedance audiometry of a B- or C-type tympanogram. Surgery was performed on patients who received medical treatment for 1--2 weeks but showed no improvement after a 2-3 month follow-up period, on patients who showed progressive retraction of the eardrum, and on patients who experienced progressive hearing loss, as shown by an increase in pure tone threshold. COM was diagnosed by the presence of symptoms, such as hearing loss, tinnitus, otalgia and/or perforation of the tympanic membrane; by the presence of otorrhea on physical examination; by soft tissue density in the middle ear or mastoid on computed tomography (CT) of the temporal bone; or by the presence of granulation tissue and/or chronic inflammation on biopsy. CholeOM was diagnosed by the presence of symptoms, including hearing loss, tinnitus, otalgia, dizziness, facial palsy, or perforation of the tympanic membrane; by the presence of otorrhea or attic destruction on physical examination; by soft tissue density in the middle ear or mastoid on temporal bone CT, or by the presence of cholesteatoma on biopsy. Patients suspected of having acute OM, head and neck anomalies, systemic diseases, or congenital or acquired immuno deficiencies were excluded.
Ethical Considerations
The purpose of the study was explained to all patients and/or their parents or guardians, with all providing written informed consent for use of patient samples. The study protocol was approved by the local institutional review board.
Samples
Prior to surgery on patients with exudative OM, the external auditory canal was washed with potadine solution, a radial incision was made in the anterior inferior quadrant of the tympanic membrane, and a tympanostomy tube was inserted. Middle ear effusion fluid (MEEF) was aseptically aspirated using a collector (Xomed Trace Products, Jacksonville, FL, USA) while avoiding bleeding. Granulation tissue was obtained from patients with COM and cholesteatoma was obtained from patients with CholeOM during tympanomastoidectomy. Samples were transferred to Eppendorf tubes and stored at -80 °C.
RNA extraction and real-time PCR procedures
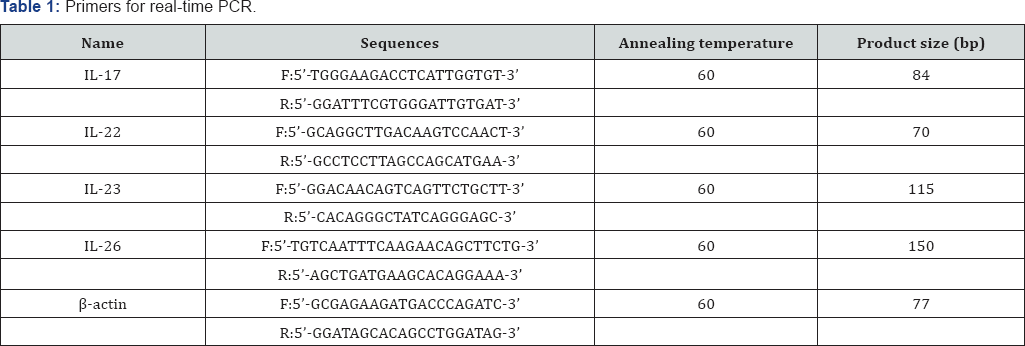
RT-PCR: reverse transcriptase-polymerase chain reaction, F: forward; R: reverse; bp: base pairs, IL: interleukin.
Total RNA was extracted from patient samples using TRIzol reagent, according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). RNA was isolated from skin samples using RNeasy Mini kits (Qiagen, QIAcube, Hilden, Germany). First-strand cDNA was synthesized from 1μg of total RNA using a reverse transcription system with random hexamers (Promega, Madison, WI, USA) according to the manufacturer's protocol. Real-time PCR was performed on a StepOnePlus realtime PCR system. Each 20μl reaction mixture contained 1μl of cDNA, 10μl of Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 2μl of specific primers (Table 1), and 7μl of PCR-grade water. The amplification protocol consisted of an initial denaturation at 95 °C for 10min, followed by 40 cycles of denaturation at 95 °C for 15s and annealing and extension at 60 °C for 1min. The crossing points of the target genes with βactin were calculated using the formula 2-(target gene-βactin), and relative amounts were quantified.
Bacterial culture
Effusion fluid samples from patients with OME and otorrhea samples from patients with COM and Chole OM were obtained using sterile cotton swabs (Xomed Trace Products, Jacksonville, FL, USA), which were immersed in Stuart transport medium. These samples were inoculated into solid blood agar and liquid thioglycollate medium (Hangang, Kun-po, Korea), and the cultures were incubated for 24 h at 35 °C. Bacteria that formed colonies were identified by Gram staining and biochemical testing.
Statistical analysis
All data were analyzed statistically using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL, USA). Differences between groups were determined using the Mann-Whitney U-test or the Kruskal-Wallis test, followed by post hoc comparisons (Tukey honest significant difference test and Bonferroni-adjusted Mann-Whitney test). Statistical significance was set at p<0.05.
Results
The 87 patients enrolled in this study included 51 males and 36 females. Bacteria were detected in samples from 38.5% of patients with OME, 70.8% of patients with COM, and 79.2% of patients with CholeOM. Of these three groups, 15.4%, 29.2%, and 45.8%, respectively, underwent revision surgery of the patients in the OME, COM, and CholeOM groups, 100%, 58.3%, and 54.2%, respectively, had conductive type hearing loss and 0%, 41.7%, and 45.8%, respectively, had sensorineural type hearing loss (Table 2). media.
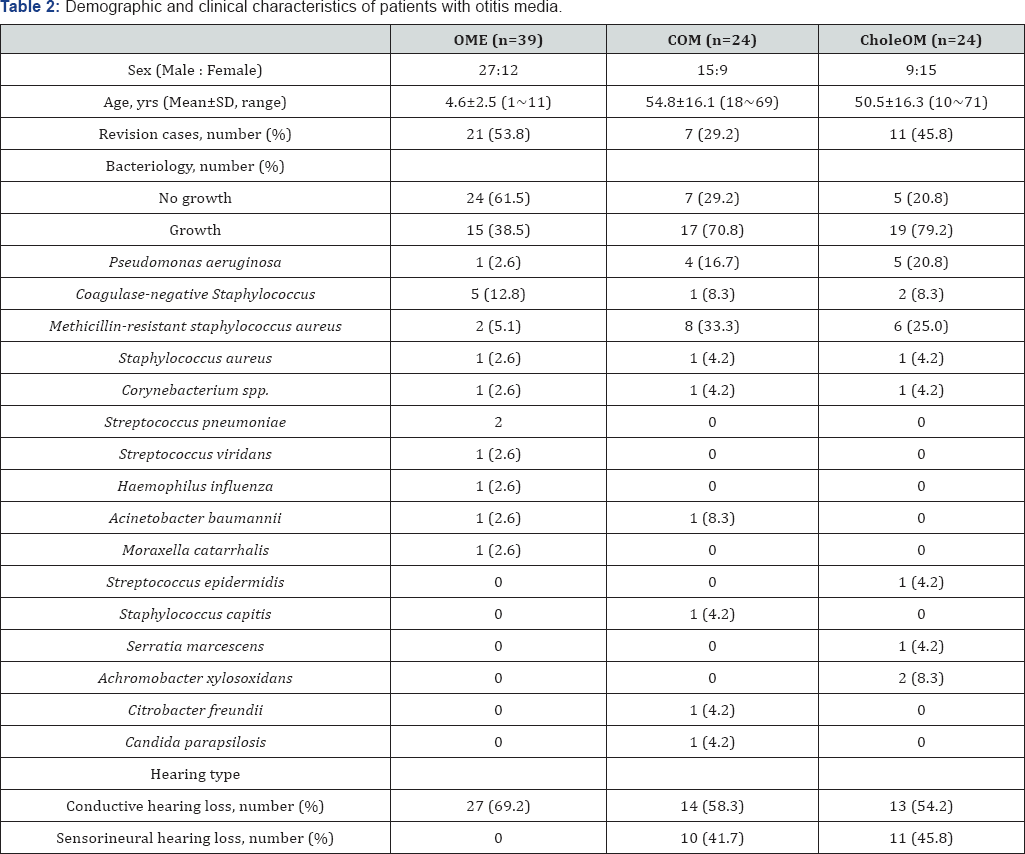
OME: otitis media with infusion; COM: chronic otitis media; CholeOM: cholesteatomatous otitis media.
The levels of expression of IL-17, IL-22, IL-23, and IL-26 mRNAs were all higher in patients with OME than in patients with COM and CholeOM (p<0.05), although there were no significant differences between patients with COM and patients with CholeOM (p>0.05) (Figure 1). Similarly, a comparison of bacteria-positive samples from the three groups showed that the levels of expression of IL-17, IL-22, IL-23, and IL-26 mRNAs were all significantly higher in the OME group than in the COM and CholeOM groups (p <0.05) with no significant differences between the COM and CholeOM groups (p > 0.05) (Figure 2). Moreover, comparisons of the levels of IL-17, IL-22, IL-23, and IL-26 mRNAs in patients with conductive hearing loss (Figure 3) and with sensorineural hearing loss (Figure 4) showed that, in both subsets, the levels of expression of these four mRNAs were significantly higher in patients with OME than in patients with COM and CholeOM (p <0.05), with no difference between the latter two groups (p > 0.05).


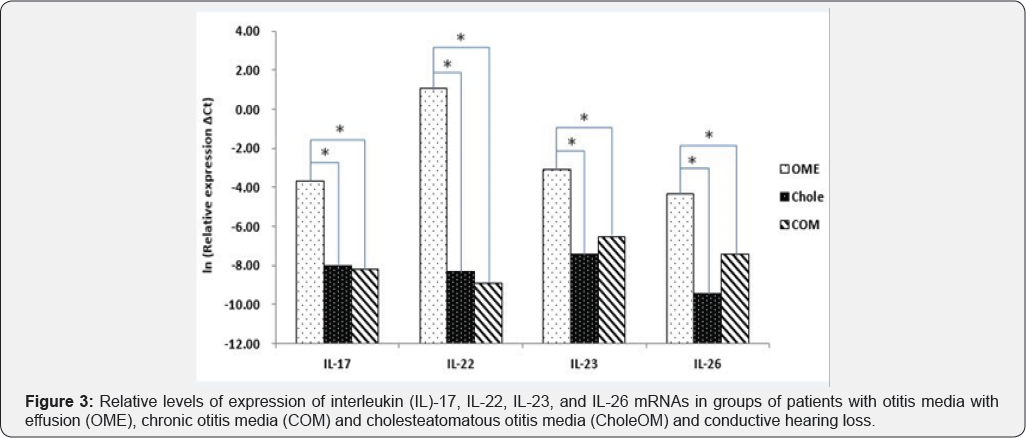
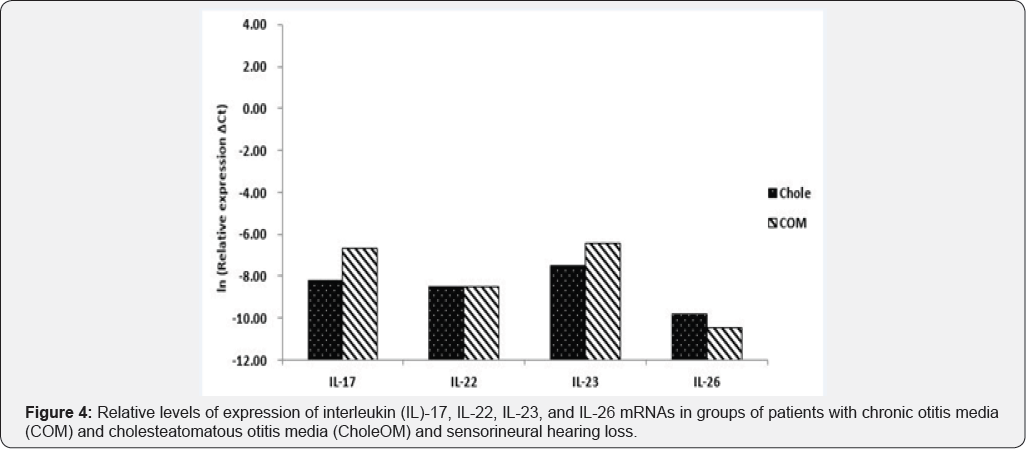
Discussion
Among the primary causes of OM are bacterial and viral infections. Host defenses against pathogens are initially triggered by the innate immune system, followed by the adaptive immune system during later stages of infection. Cytokines are responsible for cell-to-cell signaling during immune responses. Among the 200 cytokines identified to date are 35 types of interleukins, 48 types of chemokines, two groups of interferons, colony stimulating factors, growth factors, and tumor necrosis factor (TNF). However, few studies have analyzed the roles of these cytokines or their association with other immune cells in the prevention or treatment of OM [10,11].
The present study analyzed the levels of expression of mRNAs encoding four types of cytokines, IL-17, IL-22, IL-23, and IL-26. Previously, IL-17 was found to play a role in immune mechanisms against pneumococcal OM and was elevated in the middle ear effusion fluid of pediatric patients with OME [12, 13]. In addition, IL-17 has been shown to be associated with bone damage, playing a significant role in the pathogenesis of rheumatoid arthritis, which is characterized by chronic inflammation in the synovial tissues of multiple joints [14]. In addition to being expressed in patients with exudative OM, IL-17 is believed to induce bone destruction in patients with CholeOM by activating various cytokines and enzymes. Although this finding suggests that the level of IL-17 expression is elevated in CholeOM patients with severe osteoclasia, we found that the levels of IL-17 mRNA did not differ significantly between our OME and COM groups.
IL-22 is highly expressed by Th17 cells and is closely associated with chronic inflammation. Depending on the characteristics of inflammatory responses, the inflammatory and protective effects of IL-22 within tissues are bi-functional [15,16]. For example, IL-22 was found to be involved in the defenses against pneumococcal OM and its expression is elevated in MEEF of pediatric patients with OME [13]. Prior to the present study, however, the expression of IL-22 was not investigated in patients with COM and CholeOM. We found that IL-22 mRNA was expressed in patients with OME, COM and CholeOM, suggesting that IL-22 has particular immunological roles in the pathogenesis of these conditions.
IL-23, which is expressed chiefly by macrophages and dendritic cells (DCs), plays major roles in autoimmunity, chronic bowel inflammation, joint disease, and inflammatory joint disease such as ankylosing spondylitis [17,18]. IL-26 is produced when primary T cells, NK cells, and T cell clones are stimulated by specific antigens or mitogenic lectins. Activated Th17 cells express IL-26, along with IL-17 and IL-22, and the level of expression of IL-26 mRNA has been reported to be increased in inflamed colonic tissue from patients with inflammatory bowel disease [19,20]. However, the associations of IL-23 and IL-26 with OM remain unknown.
The results of this study, showing that IL-17, IL-22, IL-23 and IL-26 mRNAs were expressed in patients with OME, COM and CholeOM, suggest that these cytokines are involved in the pathophysiology of these three types of OM. The higher levels of these mRNAs in patients with OME than in patients with COM and CholeOM may be due in part to OME being a more acute type inflammation. In addition, inflammation and immune responses may be more active in the effusion fluid of patients with OME than in tissues from patients with COM and CholeOM.
The higher levels of expression in patients with OME than in patients with COM and CholeOM were observed in samples both positive and negative on bacterial culture, as well as in patients with conductive hearing loss. These findings indicated that the higher levels of IL-17, IL-22, IL-23 and IL-26 mRNAs in patients with OME were unrelated to the presence or absence of bacteria or to the presence of conductive hearing loss. A similar comparison was not possible in patients with sensorineural hearing loss, as no patient with OME experienced this type of hearing loss. A comparison of patients with COM and CholeOM and sensorineural hearing loss found no significant differences in the levels of expression of IL-17, IL-22, IL-23 and IL-26 mRNAs. This lack of difference may be due to three possible reasons. First, COM and CholeOM are both chronic diseases. Second, the inflammation and immune responses of the four cytokines within inflammatory tissues in middle ear cavity may constitute similar defense mechanisms. Third, the four cytokines may have no relationship with conductive or sensorineural hearing loss, indirectly suggesting that cytokines have no effect on lesions in the ossicles, oval window, and round window.
This study had several limitations. First, the lack of a control group prevented comparisons of immune responses within the middle ear between patients with OM and normal controls. For ethical reasons, MEEF, rather than middle ear mucosa, was tested in patients with OME. Second, this study measured levels of expression of cytokine mRNAs rather than proteins. Results obtained by measuring cytokine concentrations may have differed, as some of these mRNAs may not be translated. Third, our use of conventional bacterial culture rather than PCR to enhance the sensitivity of detection may have affected the overall bacterial detection rate. Fourth, prior treatment with antibiotics of patients with early stage OM, otorrhea or inflammation may have affected the levels of expression of IL-17, IL-22, IL-23 and IL-26 mRNAs. Finally, as these patients had long-term OME, COM and CholeOM, complex interactions among innate and adaptive immunity and other inflammatory responses within the middle ear cavity had already occurred. These responses may have affected the expression of cytokine mRNAs.
Conclusion
IL-17, IL-22, IL-23, and IL-26 mRNAs are involved in the pathophysiology of OM. The levels of all four mRNAs are significantly higher in patients with OME than in patients with COM and CholeOM, although there were no significant differences between the latter two groups.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2011-0030072).
References
- Ali Q, Tan L, Katherine B, John PB, Matija D (2014) Update on otitis media-prevention and treatment. Infect Drug Resist 7: 15-24.
- Rovers MM, Schilder AG, Zielhuis GA, Rosenfeld RM (2004) Otitis media. Lancet 363(9407): 465-473.
- Teele DW, Klein jO, Chase C, Menyuk P, Rosner BA (1990) Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. Greater Boston Otitis Media Study Group. j Infect Dis 162(3): 685-694.
- Mittal R, Kodiyan J, Gerring R, Mathee K, Li JD, et al. (2014) Role of innate immunity in the pathogenesis of otitis media. Int J infect Dis 29: 259-267.
- Semann MT, Megerian CA (2006) The pathophysiology of cholesteatoma. Otolaryngol Clin North Am 39(6): 1143-1159.
- Olszewska E, Wagner M, Bernal-Sprekelsen M, Ebmeyer J, Dazeert S, et al (2004) Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol 261(1): 6-24.
- Mittal R, Robalino G, Gerring R, Chan B, Yan D, et al. (2014) Immunity genes and susceptibility to otitis media: a comprehensive review. J Genet Genomics 41(11): 567-581.
- Juhn SK, Jung MK, Hoffman MD, Drew BR, Preciado DA, et al. (2008) The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolary 1(3): 117-138.
- Smirnova MG, Birchall JP, Pearson JP (2004) The immunoregulatory and allergy-associated cytokines in the aetiology of the otitis media with effusion. Mediators Inflamm 13(2): 75-88.
- Zhang JM, An J (2010) Cytokines, Inflammation and Pain. Int Anesthesiol C 45(2): 27-37.
- Johnson MD, Fitzgerald JE, Leonard G, Burleson JA, Kreutzer DL (1994) Cytokines in experimental otitis media with effusion. Laryngoscope 104(2): 191-196.
- Habets MN, van Selm S, van Opzeeland FJ, Simonetti E, Hermans PW, et al. (2016) Role of antibodies and IL17-mediated immunity in protection against pneumococcal otitis media. Vaccine 34(48): 59685974.
- Kwon OE, Park SH, Kim SS, Shim HS, Kim MG, et al. (2016) Increased IL-17 and 22 mRNA expression in pediatric patients with otitis media with effusion. Int J Pediatr Otorhinolaryngol 90: 188-192.
- Mclnnes IB, Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7(6): 429-442.
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, et al. (2008) Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29(6): 947-957.
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, et al. (2008) Interleukin-2 2 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14(3): 282-289.
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, et al. (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421(6924): 744-748.
- Duvallet E, Semerano L, Assier E, Falgarone G, Boissier MC (2001) Interleukin-23: a key cytoline in inflammatory disease. Ann Med 43(7): 503-511.
- Dambacher J, Beigel F, Zitzmann K, De Toni EN, Goke B, et al (2009) The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut 58(9): 1207-1217.
- Donnelly RP, Sheikh F, Dickensheets H, Savan R, Young HA, et al. (2010) Interleukin-26: an IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev 21(5): 393-401.





























