Novel Eustachian Tube Dysfunction Management Techniques: Prototype Feasibility Studies in Fresh Cadaveric Specimens
Jagdish Chaturvedi1*, Abu Saquib Tauheed2, Pooja Kadambi3, Aboli Joshi4, Gaurika Singhal4 ,Tyler John Dougan4, Eleanor Glockner4, Mohan Jagade5, Ravi Jangir1, Rohan Dsouza1, Darien Rodrigues1, Rishabh Sirdesai1and Parul Chachra1
1ENTConsultant, Fortis Hospital, India
2Affordable Invention in MedTech (AIM) Fellow, India
3Lead System Designer, InnAccel Technologies Pvt Ltd, India
4Interns, InnAccel Technologies Pvt Ltd,, India
5Professor and Head of Department, ENT and Head and Neck Surgery, India
Submission: March 21, 2018; Published: April 25, 2018
*Corresponding author: Jagdish Chaturvedi, Department of Otorhinolaryngology, India- 560 076, Tel: +91-9650928582,Email: dr.jagdishc@jrmail.com
How to cite this article: Jagdish C, A Saquib T, Pooja K, Aboli J, Gaurika S, et al. Novel Eustachian Tube Dysfunction Management Techniques: Prototype Feasibility Studies in Fresh Cadaveric Specimens. Glob J Otolaryngol 2018; 15(1): 555902. DOI: 10.19080/GJO.2018.15.555902
Abstract
Background: The underlying etiology and natural history of Eustachian Tube Dysfunction(ETD) is poorly understood, associated with various symptoms and can lead to a predisposition to middle-ear disease and, thereafter, permanent deafness. In developing countries, it is a bigger burden due to negligence and lack of awareness, coupled with the high cost of treatment and limited availability of dedicated effective solutions. This lead to the need to develop an effective solution for patients with chronic ETD as a day-care to avoid middle ear diseases.
Materials and Methods: Two cadaveric studies were done in March 2017 and August 2017 to test the utility and usability of various design prototypes and understand the potential barriers in managing ETD. 17 Prototypes were tested and broadly classified into three main categories as per their nature and use- Diagnostic, interventional, diagnostic & interventional.
Results: Three designs-an articulating camera, the balloon dilator and one embodiment of the expander showed promise as products to aid in the diagnosis and treatment of ETD. The electrical impedance probe had some merit as a safety feature but a more extensive study will be needed to prove its utility.
Conclusion: One prototype demonstrated a promising technique for equalization, mucus clearance, widening and possibly long-term relief for patients with ETD. The cadaver studies suggested the further consideration of three diagnostic technologies and the elimination of several others, further studies for feedback on positioning as well as physiology will be necessary. While slight variations in technology worked with some success, the other concepts showed no further promise.
Keywords: Eustachian tube dysfunction; ET; ETD; Cadaver study; Balloon dilation; Middle ear infection; Bio-Design; Inventing Medical Device
Introduction
The Eustachian tube (ET) is a complex hourglass structure which acts as connecting tube between the middle ear and the nasopharynx, and is responsible for ventilating the middle ear. [1] It also act as a pressure regulator to equalize middle ear and ambient pressure in a complex physiological phenomenon. In this study, failure in the ventilation mechanism mentioned above due to the inability of Eustachian tube to open is referred to as "Eustachian Tube Dysfunction", though in general ETD is also used analogously in cases of patulous eustachian tube. The 1996 World Development Report estimated that 2.163 million disability-adjusted life-years were lost due to Otitis media, of which developing world contributed to 94% of the morbidity [2]. Prevalence surveys have shown a global burden of 65-330 million individuals with draining ears due to middle ear infections , 60% of whom (39-200 million) suffer from significant hearing impairment [3]. Over 90% of this burden is borne by developing countries. Middle ear infection is uncommon in the developed countries like Australia, Middle East, Americas and Europe [3].
The underlying etiology and natural history of ETD is poorly understood [4]. There is a lack of clear diagnostic criteria [5], which further impairs our ability to study the disease and explore potential therapies. Nasal steroid sprays or anti-reflux therapies are often used as first-line options, though there is a lack of clinical data to back its effectiveness. No significant differences were found between treatment option and placebo during a randomized placebo-controlled trial with nasal steroid sprays [6].
Similarly, a recent systematic review found no statistically significant improvement with any interventions including observation, nasal steroids, and various surgical techniques [7]. In select patients there is redundant mucosa in the area at the pharyngeal end of the ET, thus impairing its dilation. Ablation of this tissue with lasers [8] or microdebriders [9] has shown promise in small studies, but these interventions are not appropriate in all patients. Other novel therapies have focused on the cartilaginous portion of the ET [10]. A recent, promising innovation, balloon dilation, is known as tuboplasty [11]. Balloon Eustachian Tuboplasty has limited data in India regarding the availability or efficacy of this technique. However, there is a high burden of morbidity in India due to the large number of patients suffering with ETD [12].
The need to develop a solution for ETD came out of a modified process research approach [13] designed to identify need specifically for Indian healthcare system based on Stanford Bio-Design process [14] which is a study dedicated to identifying unmet needs in the various field of medical science. The design and execution of these cadaver studies and the wide array of experiments demonstrates a useful approach for creating qualified medical device solutions for diagnostic and therapeutic purposes for the ET. There are limitations related to assessing the efficacy and true safety because of the nature of the cadaver model and variances in live patients.
Materials & Methods
The study was conducted in the Simulation Lab of MS Ramaiah Advanced Learning Center, Bangalore, India. The study was done in 2 phases on 30th March 2017 and 10th August 2017. The Simulation Lab of MR Ramaiah Advanced Learning Center has an operation theatre like setup with endoscopic and surgical instruments, overhead lighting, headlamps, and consumables. Jagdish Chaturvedi, ENT consultant, Fortis Hospital, Bangalore was the principal investigator and overseeing physician for both cadaveric studies, Dr Mohan Jagade, Professor and Head of Department, ENT and Head and Neck Surgery, JJ Hospital, Mumbai was also involved in the first study. Pooja Kadambi (Lead Systems Engineer at Innaccel), Gaurika Singhal and Aboli Joshi (Design Interns NID) were also involved in these studies.
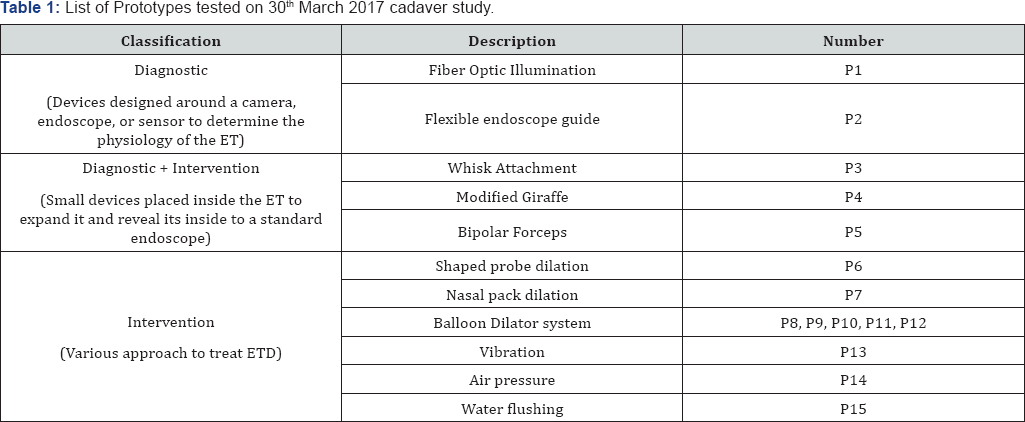
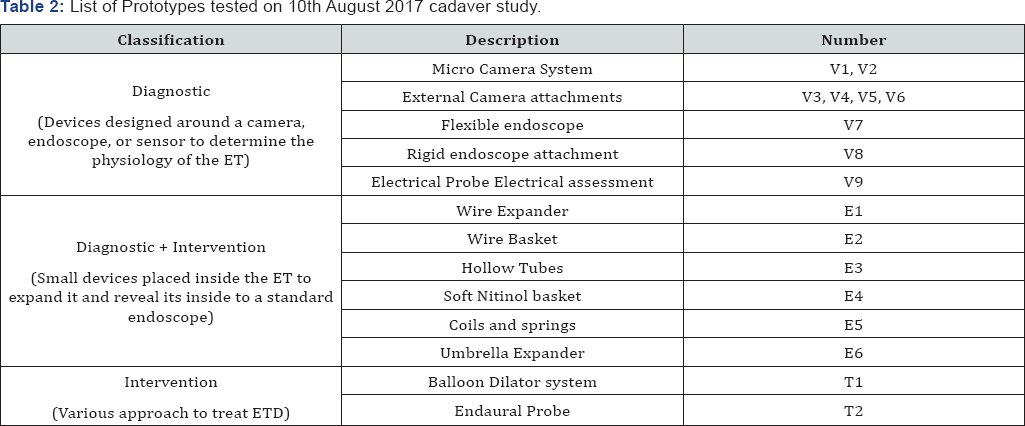
Prior to the study, training was conducted for all non-medical personnel. The training was conducted by Jagdish Chaturvedi and included relevant anatomy, cadaveric study protocols and medical procedures training (endoscopy, balloon dilation of eustachian tube and tympanostomy). The study was carried out on fresh cadavers, non-frozen within 36 hrs with all the internal anatomy intact. Table 1 & 2 list the various prototypes evaluated on 30th March and 10th August cadaveric study.
ET - Eustachian Tube; ETD - Eustachian Tube Dysfunction
Once the cadavers were cleaned and verified with a diagnostic endoscopy the prototypes were tested by the principal investigator and monitored for usability, utility, and safety (injury). The order and priority for testing various prototypes were given based on least to most damaging, and most likely to work to least likely to work. Measurements of the nasopharynx were taken with a metal tube probe. A septoplasty was performed on the cadaver in March 2017 to allow for access because the septal spur was blocking access to the Eustachian tube on one side Table 3.
ET - Eustachian Tube; ETD - Eustachian Tube Dysfunction

Results
ET - Eustachian Tube; ETD - Eustachian Tube Dysfunction
ET - Eustachian Tube; ETD - Eustachian Tube Dysfunction
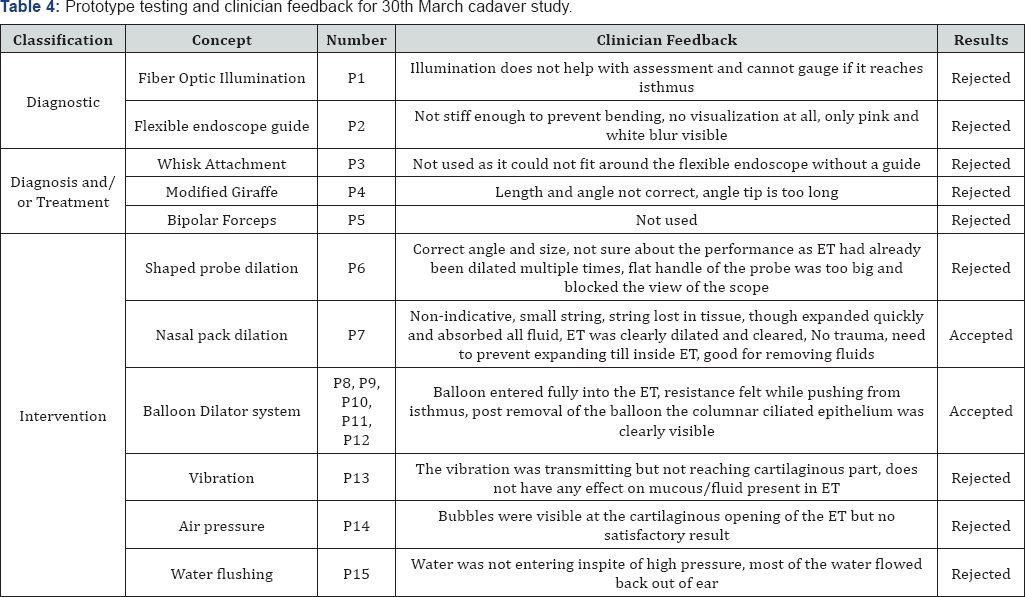
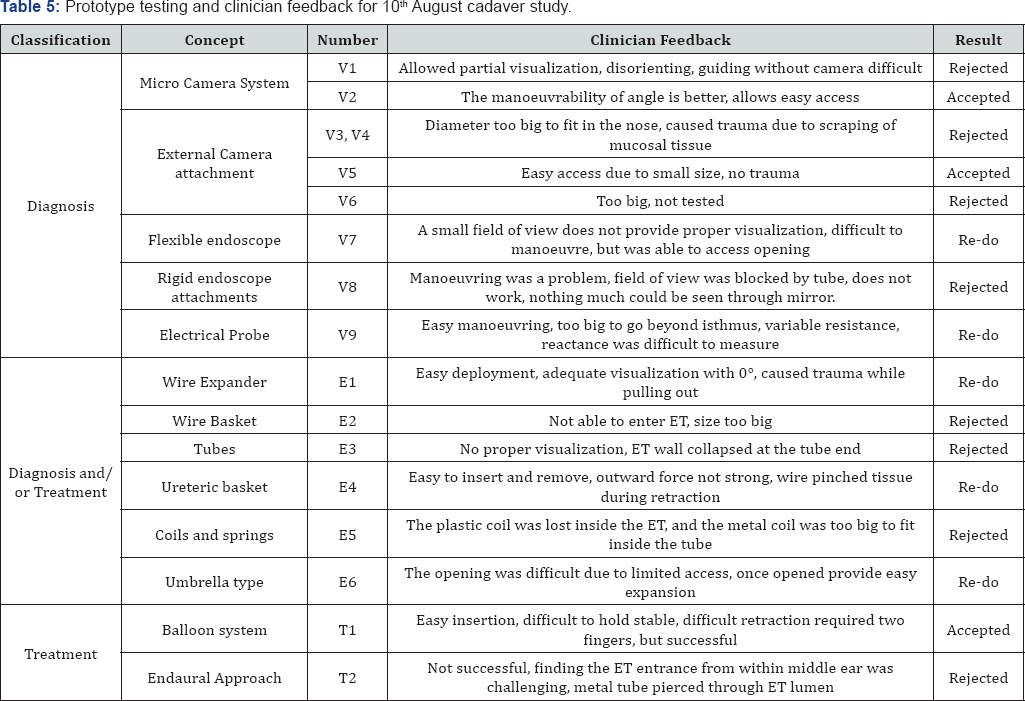
Table 4 & 5 lists the clinician feedback and decisions taken for all tested prototypes on 30th March and 10th August 2017 respectively.
Description of result criteria
Accepted: Prototypes performed adequately for ETD management with no significant trauma or usability issues.
Rejected: Prototypes caused significant trauma and usability issues with failure in performing the intended function.
Re-do: Prototypes causing no significant trauma but having slight usability issues which can be improved with changes in dimensions or technology.
Discussion
The use of fresh cadavers in a surgical simulation setting was beneficial as it offered a real world usability scenario in terms of human anatomy, tactile feedback of tissue and the dangers of potential injury. The compliance of tissues and the ease or difficulty in separating one structure from another resembles those of living tissue as compared to animal or synthetic models [15].
Prototypes P1 to P15 were evaluated for their utility and usability in the first cadaver study conducted on 10th March 2017. Prototypes P1, P2, P3, P4, P5, P6, P13, P14, and P15 were rejected as they had minimal or no effect on mucosal removal and/or opening the ET obstruction. The visualization prototypes were also unable to provide adequate visualization or proper access to ET.
Balloon-based dilation prototypes P8, P9, P10, P11 and P12 showed some promising results in the dilation and management of ETD. The prototypes P8 to P12 produced adequate dilation without causing trauma. It was decided to build second- generation balloon-based prototypes for further investigation and trials. Prototype P7 also produced adequate and quick expansion but it was non-indicative and also led to an adverse event due to the loss of string in the mucosal tissue. It was acceptable for thin fluid absorption and medication delivery.
The second generation of prototypes based on balloon system and 16 other concepts were developed and tested in cadaver studies conducted on 10th August 2017. Prototypes V1, V3, V4, V6, V8, E2, E3, E5, and T1 were rejected due to the lack of safety (causing trauma) and utility. The rejections were based on the trauma caused during the procedure, the difficulty in access during insertion and retraction, the small field of view and the inability to dilate or visualize the ET for diagnostic purposes. The expanders did not have sufficient therapeutic impact to be considered as an interventional solutions and the risk of injury outweighed any potential temporary benefit of pressure equalization using rigid expanders.
Prototypes V2, V5, and T1 were found to be acceptable during the cadaver study. All three prototypes worked perfectly in the study and performed as per the intended design. The selection criteria was based on ease to use, ease of access of the ET, absence of trauma during insertion, adequate visualization of anatomy and landmarks, allowed for adequate expansion of tissue. Prototype V5 and V2 were also used together as a combination to attempt a hybrid approach, it allowed for adequate visualization for the diagnosis. It showed significant promise and can be further refined and applied for diagnostic purposes. Prototypes V7, V9, E1, E4, and E6 required further testing to prove their usability and utility. Prototypes that were suggested for rework showed promising results in one or more of the acceptance criteria but were either dysfunctional or nonfunctional. Prototype V7 and V9 provided ease of access but inadequate visualization, E1 provided adequate visualization, easy deployment but led to untoward trauma during retraction, E4 allowed for ease of access but also led to trauma during retraction and E6 provided adequate expansion of ET post access but difficult access the ET.
Study Limitations
The most significant shortcoming of these cadaver studies was the inability to quantify the depth visualization provided by the different cameras and scope-based designs. It would be beneficial to have a system in place to measure the visible distance anterior to the camera or the endoscope, such as a ruled or marked guide inserted into the ET, in future studies. The pain, discomfort and bleeding associated with the prototypes could not be objectively assessed in a cadaver especially given the high sensitivity of the ET during endoscopic examination in live patients, usually done under local anaesthesia.
During evaluation of some designs, such as the wire expanders, it was difficult to objectively assess the tissue damage due to expansion. While it was obvious that the sharp ends posed a serious danger, there was no visually evident damage to the ET lining. However, it is possible that this could possibly harm the mucosa of a living patient in a way that is not visible in a cadaver. This damage can be observed if the ET lining is inspected with the camera prototype immediately following removal of a wire expander. For future studies, it is recommended that a more thorough evaluation of the trauma of each expansion device is conducted.
Conclusion
The two-cadaver study suggests the recommendation of three diagnostic technologies for further consideration, as well as the elimination of several others. Prototype T1 demonstrated promise technique in pressure equalization, mucus clearance, widening and possible long-term relief and thus is the most favoured intervention. P7 could be worked on for specific interventions. The prototypes V5 and E1 in conjunction with a rigid endoscope, permitted excellent visualization of the first 10 mm of the ET, lumen which was suitable for diagnosis. Overall, better visual assessment of the inside of the ET from the nasal approach was considered to be the most appropriate diagnostic technique. The prototype V9 revealed positive results in distinguishing the isthmus from the cartilaginous ET, so it could be further studied as a system for feedback on positioning and possibly physiology. Prototypes that were rejected were characterized and documented to avoid any further exploration while further studies will be conducted for the those with successful outcomes. ET management involves a combination of suitable diagnosis, intervention and monitoring. Thus, the short listed concepts will be individually refined and possibly combined in the future to meet the ultimate need of better definitive management of chronic ETD.
Acknowledgements
The authors would like to thank InnAccel Private Limited for support in writing this article. We are also thankful to the MS Ramaiah Advanced Learning Center, Bangalore for their support in conducting these cadaver study, Stanford Seed internship program and National Institute of Design for the interns who invested their full dedication to this study. The authors are also indebted to Dr Mohan Jagade, Professor, and Head of Department, ENT and Head and Neck Surgery, JJ Hospital, Mumbai for his invaluable inputs.
Conflict of Interest
The authors are affiliated to InnAccel Technologies Pvt. Ltd.
References
- John W Seibert, Christopher J Danner (2006) Eustachian Tube Function and the Middle Ear Otolaryngologic clinics of North America 39(6): 1221-1235.
- (2004) Chronic suppurative otitis media Burden of Illness and Management Options. Switzerland: World Health Organization 23.
- Gluth MB, McDonald DR, Weaver AL, Bauch CD, Beatty CW, et al. (2011) Management of Eustachian Tube Dysfunction With Nasal Steroid Spray: A Prospective, Randomized, Placebo-Controlled Trial. Arch Otolaryngol Head Neck Surg 137(5): 449-455.
- Ali K Mahrous, Wael F Esmaeil, Mohammed H Abdel-Azim, Mohamed A Wafa (2017) Effectiveness of Balloon Tuboplasty on the Eustachian tube function: A Systematic Review Nat Sci 15(11): 124-132.
- Llewellyn A, Norman G, Harden M, Coatesworth A, Kimberling D, et al. (2014) Interventions for adult Eustachian tube dysfunction: a systematic review. 18(46): 1-80.
- Gluth MB, McDonald DR, Weaver AL, Bauch CD, Beatty CW, et al. (2011) Management of Eustachian Tube Dysfunction With Nasal Steroid Spray A Prospective, Randomized, Placebo-Controlled Trial. Arch Otolaryngol Head Neck Surg 137(5): 449-455.
- Llewellyn A, Norman G, Harden M, Coatesworth A, Kimberling D, et al. (2014) Interventions for adult Eustachian tube dysfunction: A systematic review. Health Technol Assess 18(46).?
- Caffier PP, Sedlmaier B, Haupt H, Goktas O, Scherer H, et al. (2011) Impact of laser eustachian tuboplasty on middle ear ventilation, hearing, and tinnitus in chronic tube dysfunction. Ear Hear 32(1): 132139.
- Metson R, Pletcher SD, Poe DS (2007) Microdebrider eustachian tuboplasty: A preliminary report. Otolaryngol-Head Neck Surg 136(3): 422-427.
- Kivek&3228;s I, Chao WC, Faquin W, Hollowell M, Silvola J, et al. (2015) Histopathology of balloon-dilation Eustachian tuboplasty. Laryngoscope 125(2): 436-441.
- Williams B, Taylor B A, Clifton N, Bance M (2016) Balloon dilation of the eustachian tube: a tympanometric outcomes analysis. Journal of Otolaryngology-Head & Neck Surgery 45(13).
- Manche Santoshi Kumari, Jangla Madhavi, Nagalla Bala Krishna, Koralla Raja Meghanadh, Akka Jyothy (2016) Prevalence and associated risk factors of otitis media and its subtypes in South Indian population. Egyptian Journal of Ear, Nose, Throat and Allied Sciences 17(2): 57-62.
- Herr GL (2010) Biodesign: The Process of Innovating Medical Technologies 44(5): 388.
- (2015) Biodesign Innovation Process Stanford Byers Center for Biodesign Stanford Medicine.
- Sharma M, Macafee D, Pranesh N, Horgan A F (2012) Construct Validity of Fresh Frozen Human Cadaver as a Training Model in Minimal Access Surgery. Journal of the Society of Laparoendoscopic Surgeons 16(3): 345-352.





























