Understanding Etiopathogenesis of Steven Johnson Syndrome
Sumit Sharma*
Department of ENT, Mayo Institute of Medical Sciences, India
Submission: March 14, 2018; Published: April 03, 2018
*Corresponding author: Sumit Sharma, Department of E.N.T, Mayo Institute of Medical Sciences, Gadia, Barabanki, India, Email: entsumit@rediffmail.com
How to cite this article: Sumit S. Understanding Etiopathogenesis of Steven Johnson Syndrome. Glob J Oto 2018; 14(1): 555879. DOI: 10.19080/GJO.2018.14.555879
Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe dermatologic reactions with mucocutaneous involvement that carry elevated mortality rates. They differ along a spectrum of severity based upon body surface area affected. These conditions, usually caused by a drug or infection, are believed to result from cell mediated and often drug specific cytotoxic reactions against keratinocytes, leading to widespread dermal-epidermal detachment [1]. Stevens Johnson syndrome (SJS) was first described by the two pediatricians (A. M. Stevens and F. C. Johnson) in 1922 in the New York City in two children in whom the illness was most likely triggered by infection. In 1956, A. Lyell used the term toxic epidermal necrolysis (TEN) to describe the chafed looking skin lesions in four of his patients, based on the belief that these lesions were induced by a circulating toxin. TEN was also independently described by Lang and Walker in 1956 [2].
Etio-Pathogenesis
The pathophysiological mechanism is not fully understood. It is believed to be a delayed hypersensitivity reaction mediated by Th1 cells [3] Some individuals have a genetic predisposition to develop such disorders: the so-called slow acetylators, deficient in enzymes involved in the destruction of toxic drug metabolites, such as glutathione transferase. Recently, genetic association of some HLA major histocompatibility complex alleles with the occurrence of serious drug reactions has been described. Histopathological hallmark of these diseases is widespread epidermal necrosis due to death by apoptosis of keratinocytes. CD8 cells act as mediators in this process. There are two pathways leading to apoptosis:
A. The binding of Fas (CD95), a membrane receptor present in keratinocytes, with its FasL ligand (CD95L), and the
B. Release of the perforin and granzyme B pathways.
Drugs and SJS
It is believed that drugs are the main cause of SJS (50 to 80% of cases) and TEN (around 80%), although these diseases can also be triggered by infections and malignancies [3] While drugs and cancer are more associated with adult patients, infections are the leading cause in children: it is estimated that half of patients diagnosed with SJS had a recent upper respiratory tract infection [3] Table 1.
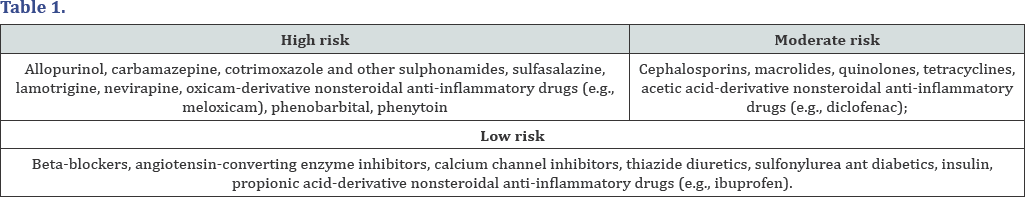
The following drugs were listed as the main ones related to the occurrence of SJS and TEN, based on RegisSCAR / EuroSCAR files [3]. In a recent international multicenter case-control study, the Euro SCAR (European Severe Cutaneous Adverse Reactions) trial, allopurinol was the most common cause of SJS and TEN, particularly when prescribed at doses equal to or higher than 200 mg per day. It is important to note that in 2014, the Food and Drug Administration (FDA) required the manufacturers of acetaminophen (paracetamol) to include SJS risk warnings in the package.
Stephen Alerhand et al. [1] suggested that potentially offending drugs can be scored upon 6 parameters by ALDEN (algorithm of drug causality for epidermal necrolysis):
a) Time delay from drug administration to reaction onset,
b) Probability of drug presence in the body,
c) Prior exposure to the same drug regardless of reaction at that time,
d) Presence of drug beyond the progression phase,
e) Drug notoriety as a cause of SJS/TEN, and the
f) Presence or absence of other etiologies.
The SJS/TEN symptoms are not clearly attributed to a drug in 20% to 25% of cases (higher for children).
Index Days
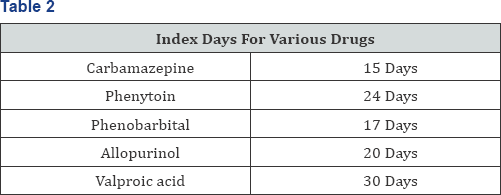
refers to the median latency time between the beginning of use and onset of SJS/TEN, The first large study to assess the risk of developing SJS or TEN distinguished between [4] Drugs used for short term periods highest risk was documented for trimethoprim sulfomethoxazole and other sulfonamide antibiotics, followed by chlormezanone, cephalosporins, quinolones and aminopenicillins. Drugs used for months or years. In this group the increased risk was confined largely to the first 2 months of treatment Table 2. The drugs include carbamazepine, followed by oxicam nonsteroidal anti inflammatory, corticosteroids, phenytoin, allopurinol, Phenobarbital and valproic acid. He further suggested that Granulysin levels are important prognostic indocators, Granulysin is a protein expressed in two isoforms (15KDa and 9KDa), produced by CD8+ Cytotoxic T cells, natural killer T cells and natural killer cells [2] Specific drug hypersensitivity leads to major histocompatibility class I restricted drug presentation and is followed by an expansion of cytotoxic T -lymphocytes, leading to an infiltration of skin lesions with cytotoxic T-lymphocytes and natural killer cells. Granulysin probably is the key mediator for disseminated keratinocyte death in SJS/TEN. Granulysin levels in the sera of patients with SJS/ TEN are much higher than in patients with ordinary drug induced skin reactions or healthy controls. Furthermore granulysin levels correlate with clinical severity. The mechanism is not IgE mediated, a desensitization of the triggering drug is not an option [4].
Non Drug Induced SJS
Infections could play a major role in triggering SJS in children, outnumbering drug induced cases in some studies. Mycoplasma pneumonia is implicated as an etiological factor in SJS, especially in children who present with mucosal lesions and limited skin involvement. Other triggers include live virus vaccinations, and DPT vaccination [2] Although Stephen Alerhand etal [4] suggests that in children, medications are the most common precipitant of SJS/TEN. The reported viral diseases include herpes simplex virus (HSV), HIV, coxsackievirus, influenza, hepatitis, lymphogranuloma venereum, and smallpox. Bacterial agents include group A beta-hemolytic streptococcus, the bacilli of diphtheria, brucellosis, typhoid fever and tularemia, mycobacteria and mycoplasma. Fungal causes include paracoccidioidomycosis, dermatophytosis and histoplasmosis. Protozoan parasites malaria and trichomonas were also related. In children, enteroviruses and Epstein Barr virus are possible causative agents. Carcinomas and lymphomas are also associated. Notwithstanding the different known etiologies, SJS is idiopathic in 25 to 50% of cases [3] Table 3.

Mycoplasma Pneumoniae and Stevens Johnson Syndrome
M. pneumoniae has been cited as the most common infectious cause of SJS. It is one of the commonest causes of atypical pneumonia. 25-33% of infected patients may show some cutaneous manifestations most notably exanthems, urticaria, and SJS. In addition to SJS, M. pneumoniae has also been noted to cause erythema multiforme (EM) and "atypical SJS" manifesting as severe mucositis without skin lesions. M. pneumoniae-associated SJS most commonly affects children and young adults compared with drug associated SJS. In younger patients, SJS may be preceded by symptoms of upper respiratory tract infection from 2 days to 2 weeks before the appearance of the rash M pneumonia induced SJS is associated with less severe complications and less internal organ involvement than those resulting from other causes. M. pneumoniae has occasionally been isolated from the involved skin of effected patients also [5] Hence a M. pneumoniae work up should be considered in patients who present with SJS, particularly if there is no history of recent medication use [6].
Genetics and Steven Johnson Syndrome
Some people with Stevens-Johnson syndrome have a genetic predisposition which increases their risk of developing the condition in response to triggering factors such as medications. The genetic variation most strongly associated with StevensJohnson syndrome occurs in the HLA-B gene. This gene is part of a family of genes called the human leukocyte antigen (HLA) complex. This complex of genes helps the immune system distinguish the body's own proteins from proteins made by foreign invaders (such as viruses and bacteria). Variations in several other HLA and non HLA genes have also been studies as possible risk factors for Stevens Johnson syndrome. However, most people with the genetic variations that increase the risk for Stevens-Johnson syndrome never develop the disease, even if they are exposed to drugs that can trigger it. It is likely that there are other factors involved in this complex process which determines whether a person ultimately develops the condition [7-9] Genetic predisposition is also linked to recurrence of SJS in a patient. In a retrospective study of 55 cases, 10 children had recurrence between 2 months and 7 years after the first episode, 3 had multiple recurrences, and 1 died.35 These findings may suggest a long lasting vulnerability and genetic predisposition for SJS/TEN [1].
HIV and Steven-Johnson Syndrome
Patients with both and medication for HIV are at increased risk for Stevens-Johnson syndrome, There is a 100 fold increase in the incidence of Stevens-Johnson syndrome in patients with HIV [4] Certain antiretroviral drugs used to treat HIV are associated with an increased risk for SJS including Viramune (nevirapine), Ziagen (abacavir), and Isentress (raltegravir). Nevirapine has been cited as the culprit in 0.3% to 1% of cases of Stevens-Johnson syndrome in HIV seropositive patients; 22 cases have been reported to the FDA since the drug was approved in 1996 [10].
References
- Stephen Alerhand, Courtney Cassella, Alex Koyfman (2016) Stevens Johnson syndrome and Toxic Epidermal Necrolysis in the Pediatric Population. Pediatr Emerg Care 32(7): 472-476.
- Sudip Das, Ramkumar Ramamoorthy (2018) Stevens Johnson Syndrome and Toxic Epidermal Necrolysis in Children 19(1): 9-14.
- Anthony Wong, An drey Augusto Malvestiti, Mariana de Figueiredo Silva Hafner (2016) Stevens Johnson syndrome and toxic epidermal necrolysis.
- Swapnil S Deore, Rishikesh C Dandekar, Aarti M Mahajan, Vaishali V Shiledar (2014) Drug Induced Stevens Johnson Syndrome. International Journal of Scientific Study 2(4).
- Rasul S, Farhat F, Endailalu Y, Tabassum Khan F, Poddar V (2012) Mycoplasma pneumoniae Induced Stevens Johnson Syndrome: Rare Occurrence in an Adult Patient. Case Rep Med 2012: 430490.
- Wetter DA, Camilleri MJ (2010) Clinical, etiologic, and histopathologic features of Stevens Johnson syndrome during an 8-year period at Mayo Clinic. Mayo Clin Proc 85(2): pp. 131-138.
- (2015) Stevens Johnson syndrome/toxic epidermal necrolysis. Genetics Home Reference (GHR).
- (2014) Stevens Johnson syndrome. MayoClinic.com.
- Foster CS (2015) Stevens Johnson Syndrome. Medscape Reference
- Warren KJ, Boxwell DE, Kim NY, Drolet BA (1998) Nevirapine associated Stevens Johnson syndrome. Lancet 351: 567.





























