Novel Anticancer Platinum and Palladium Nanoparticles from Barleria prionitis
Shalaka S Rokade1, Komal A Joshi2, Ketakee Mahajan2, Geetanjali Tomar2, Dnyanesh S Dubal3, Vijay Singh Parihar4, Rohini Kitture5, Jayesh Bellare6 and Sougata Ghosh1*
1Department of Microbiology, Modern College of Arts, Science and Commerce, Ganeshkhind, Pune-411016, India
2Institute of Bioinformatics and Biotechnology, Savitribai Phule Pune University, Pune-411007, India
3Indian Institute of Science, Education and Research, Pune-411008, India
4Department of Biomedical Sciences and Engineering, BioMediTech, Tampere University of Technology, Korkeakoulunkatu 10, 33720, Tampere, Finland
5Department of Applied Physics, Defense Institute of Advanced Technology, Girinagar, Pune-411025, India
6Department of Chemical Engineering, Indian Institute of Technology, Bombay, Powai, Mumbai-400076, India
Submission: August 18, 2017; Published: September 14, 2017
*Corresponding author: Dr. Sougata Ghosh, Department of Microbiology, Modern College of Arts, Science and Commerce, Ganeshkhind, Pune 411016, India, Email: ghoshsibb@gmail.com
How to cite this article: Rokade SS, Joshi KA, Mahajan K, Tomar G, Dubal DS, et al. Novel Anticancer Platinum and Palladium Nanoparticles from Barleria prionitis. Glob J Nano. 2017; 2(5): 555600.DOI:10.19080/GJN.2017.02.555600
Abstract
Nanomedicines are emerging outcomes of nanobiotechnology which have promising pharmaceutical applications. Although there are various physical and chemical methods for synthesis of nanoparticles, most of them involve hazardous and toxic chemicals. Thus, there is a continuously growing need for investigation of novel routes to synthesize nanoparticles with enhanced biocompatibility and reduced toxicity. Medicinal plants are rich source of diverse photochemical responsible for simultaneous reduction and stabilization of nanoparticles. Herein, we report for the first time, the synthesis of platinum nanoparticles (PtNPs) and palladium nanoparticles (PdNPs) using Barleria prionitis leaf extract (BPLE). PtNPs and PdNPs were characterized using UV-visible spectroscopy.
High resolution transmission electron microscopy (HRTEM) revealed that the PtNPs were between 1 to 2 nm while PdNPs were between 5 to 7 nm. Further energy dispersive spectroscopy (EDS) and dynamic light scattering (DLS) confirmed the elemental composition and hydrodynamic size, respectively. Fourier transformed infrared spectra (FTIR) confirmed the involvement of diverse photochemical in reduction and stabilization of the nanoparticles. Both PtNPs and PdNPs were tested for anticancer activity against human breast adenocarcinoma (MCF-7) cell lines which showed reduced viability up to 60.08 ± 2.4 % and 57.22 ± 1.68 %, respectively.
Further, flow cytometric studies and confocal microscopy using dual staining method with annexin V-FITC and propidium iodide indicated apoptosis induction as the plausible mechanism exhibiting externalization of phosphatidylserine and loss of cell membrane integrity. This is the first report on PtNPs and PdNPs synthesized by BPLE as promising anticancer agents.
Keywords:Barleria prionitis; Platinum nanoparticles; Palladium nanoparticles; Anticancer; Flow cytometry; Confocal microscopy
Introduction
Biological synthesis of nanoparticles has recently emerged as a promising field of research owing to large scale applications in both biotechnology and nanotechnology [1] Synthesis of nanoparticles with well defined size and shape is critical for their activity [2-4] Although various physical and chemical methods like supercritical fluid synthesis, plasma or flame spraying, sol-gel process, laser pyrolysis, aerosol based process, chemical vapour deposition, atomic or molecular condensation, mechanical milling, chemical etching, electro-explosion, sputtering and laser ablation are extensively used to synthesize monodispersed nanoparticles, biological routes have gained considerable attention in the past decade [5-9].
The use of hazardous chemicals, toxic solvents, high temperature and high pressure in the synthesis procedure limit their applications and poses threat to the environment [10]. Hence, there is a need for development of environmentally benign, non-toxic, eco-friendly rapid methods for nanoparticle synthesis. In view of the background, there is a growing need to design novel biological routes for synthesis of monodispersed nanoparticles for various physico-chemical and biological applications. In spite of being safe, cost-effective, sustainable and eco-friendly, microbial synthesis has potential drawbacks like maintenance of sterile conditions, cell mass and size control. Hereby, medicinal plants are considered as efficient sources of photochemical that would not only reduce but also stabilize the bioreduced nanoparticles [11-16].
As a part of our growing interest we have reported on optimized processes for synthesis of gold, silver, copper, platinum and palladium nanoparticles using various medicinal plants like Dioscorea bulbifera, Dioscorea oppositifolia, Plumbago zeylanica, Gloriosa superba, Gnidia glauca [17-24].
Barleria prionitis is one of the most widely used traditional complementary and alternative medicine. It is reported to have curative effect against whooping cough, gout, respiratory problem, toothache, pyorrhoea, scabies, purulent infections, cataract, glandular swellings, boils, leucoderma, sciatica, dropsy, mouth ulcers, dysuria, jaundice, snake bite, edema and asthma. Its pharmacological significance further includes antimicrobial, anthelmintic, antifertility, antioxidant, antidiabetic, antiinflammatoty, anti-arthritic, cytoprotective, hepatoprotective, diuretic, antidiarrhoeal, enzyme inhibitory and anti-nociceptive activities without any toxic effects [25].
Various phytochemicals like balarenone, pipataline prionisides, barlerinoside, verbascoside, shanzhiside methyl ester, barlerin, acetylbarlerin, lupulinoside, scutellarein are isolated and identified as its bioactive principles [26]. Recently, we have reported on its nanobiotechnological potential by synthesizing gold nanoparticles and silver nanoparticles using B. prionitis leaf extract (BPLE) [21]. However, till date there are no reports on synthesis of platinum (PtNPs) and palladium (PdNPs) nanoparticles using BPLE. In view of this background, herein we report for the first time, synthesis of PtNPs and PdNPs using BPLE. Further the bio reduced nanoparticles were checked for the anticancer activity against breast cancer cells (MCF-7). The mechanism of anticancer activity was also established using flow cytometry and confocal microscopy.
Materials and Methods
Plant material and preparation of extract
BPLE was prepared as reported earlier [21]. In short, B. prionitis leaves were collected from Western Ghats of Maharashtra, India, which were thoroughly washed and shade dried for 2 days at room temperature. The dried leaves were pulverized using an electric blender into fine powder, 5 g of which was taken in a 300 mL Erlenmeyer flask with 100 mL of distilled water and boiled for 5 minutes before final decantation and filtration through Whatman No.1 filter paper. The filtrate was collected and stored at 4 °C for further use.
Synthesis of PtNPs and PdNPs and UV-vis spectroscopy
PtCl6 2- ions were reduced by addition of 5 mL of BPLE to 95 mL of 10-3M aqueous H2PtCl6, 6H2O solution. Similarly, PdNPs were synthesized by addition of 5 mL of BPLE to 95 mL of 10- 3M aqueous PdCl2. Synthesis of PtNPs and PdNPs were achieved by carrying out the reaction at 100°C for 5 hours which was monitored by UV-Vis spectroscopy on a spectrophotometer (Spectra Max M5, Molecular Devices Corp, USA) operated at resolution of 1 nm.
High resolution transmission electron microscopy (HRTEM), energy dispersive spectroscopy (EDS), dynamic light scattering (DLS)
Analysis of size and shape of bioreduced PtNPs and PdNPs were determined using JEOL-JEM-2100 higher resolution transmission electron microscope (HRTEM) equipped with energy dispersive spectrometer (EDS) at an energy range 0-20 keV. Particle size was analyzed using the dynamic light scattering equipment (Zetasizer Nano-2590, Malvern Instruments Ltd, Worcestershire, UK) in polysterene cuvette.
Fourier Transform Infrared (FTIR) Spectroscopy
PtNPs and PdNPs synthesized after 5 hours of reaction were centrifuged at 10,000 rpm for 15 minutes at room temperature, following which the pellet was re-dispersed in sterile distilled water to remove any plant materials. The process of centrifugation and re-dispersion in sterile distilled water was repeated thrice to ensure better separation of free entities from the nanoparticles. The purified pellet was then dried and subjected to FTIR (IRAffinity-1, Shimadzu Corp, Tokyo, Japan) spectroscopy measurement using the potassium bromide (KBr) pellet technique in the diffused reflection mode at a resolution of 4 cm-1. Nanoparticle powders were mixed with KBr and subjected to IR source 500-4000 cm-1. Similar process was used for the FTIR study of BPLE before and after bioreduction.
Ant proliferative activity
In order to compare the cytotoxic effect of PtNPs and PdNPs, MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) assay was performed as per our earlier methods [27-29] 4 x 104 cells were seeded per well in a 96-well plate. The nanoparticles were added to the cells at a concentration of 200 μg/ml after 24 hours of seeding the cells. Media was removed after 48 hours, and the cells were washed with PBS. MTT (0.5mg/mL) was added to the wells and incubated for 3 hours. The formazan crystals were dissolved using acidified isopropanol and the absorbance was measured at 570 nm. The statistical analysis was done by using One Way ANOVA.
Flow cytometric analysis
In order to confirm the mechanism behind cell death, flow cytometric analysis of cells treated with respective nanoparticles was performed as reported earlier. In short, 5×105 cells were seeded in a T-25 flask and after 24 hours these cells were treated with PtNPs and PdNPs nanoparticles at a concentration of 200 μg/ml. After 48 hours of treatment, the cells were harvested and stained with Annexin-V-FITC (dilution 1:20) and propidium iodide (dilution 1:20) for 10-15 minutes at 4oC. These were then acquired using BD FACS Verse and analysed by BD FACS Suit software.
Confocal microscopy
The flow-cytometry data was supported by immunofluorescence staining. MCF-7 cells were seeded on to glass cover slips at a density of 5×104 cells. After 48 hours of treatment with 200 μg/ml of PtNPs and PdNPs, the cells were then stained with Annexin-V(AV)-FITC and PI, both at a dilution of 1:20 for 15 minutes at 4°C followed by observation under LSM 780 confocal laser scanning microscope, Carl Zeiss.
Results
UV-visible spectroscopy
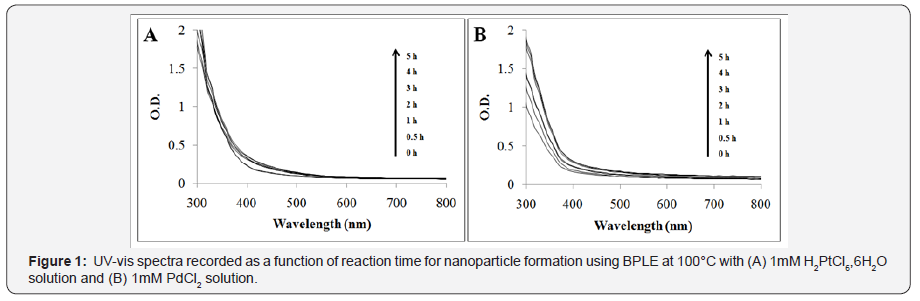
Synthesis of PtNPs was indicated by the colour change on addition of BPLE into 1mM H2PtCl6, 6H2O solution. Initially the colour changed into light brown which on incubation at 100°C under shaking turned into intense dark brown. Similarly, UVvisible spectroscopy exhibited the enhancement of the intensity of the on incubation for 5 hours (Figure 1A). The synthesis was found to be rapid and the intensity increased sharply from 0 hour to 1hour followed to which no distinctive rise was observed even though the spectra was recorded at regular intervals till 5 hours. This shows that maximum synthesis was completed within 1 hour. In case of PdNPs as well, addition of BPLE into 1m M PdCl2 solution showed development of dark brown colour which gradually turned into black on incubation till 5 hours at 100°C under shaking. Beyond 5 hours no visible colour change indicated the complete reduction of the Pd salt to PdNPs. Further UV-visible spectroscopy confirmed the synthesis of PdNPs by exhibiting the gradual increase in the intensity of the spectra from 0 to 5 hours (Figure 1B). Beyond 3 hours the increase in the spectral intensity was comparably low indicating the maximum synthesis of PdNPs within 3 hours.
HRTEM, EDS, DLS analysis
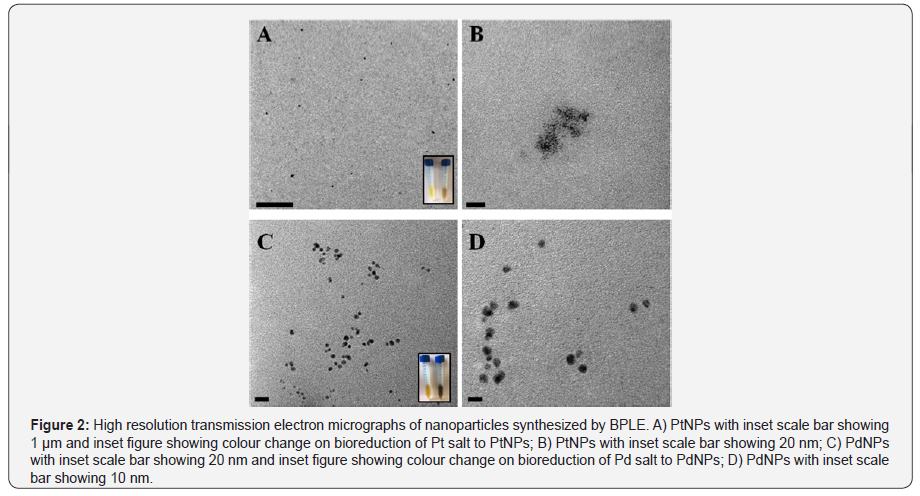
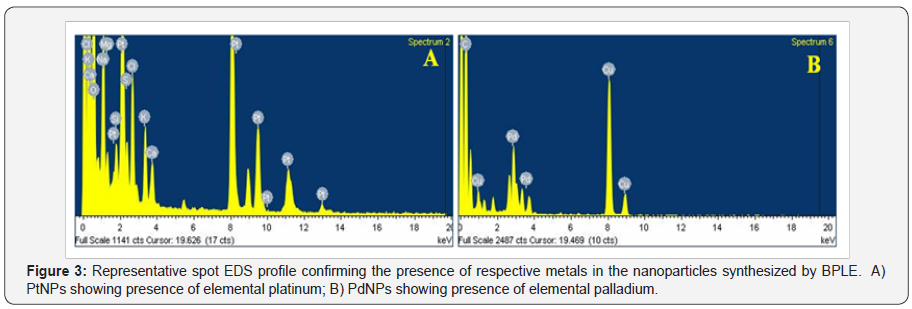
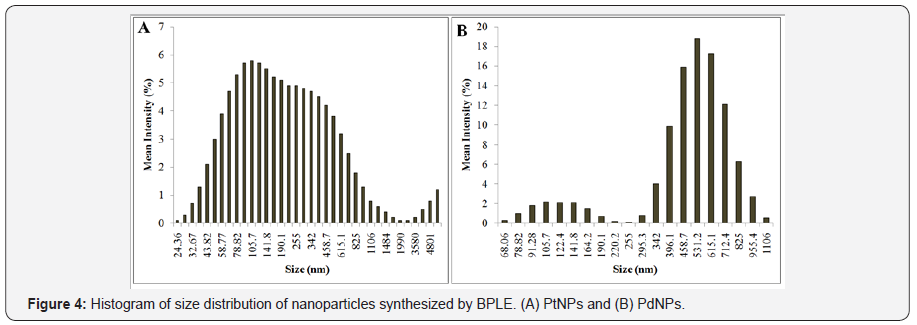
PtNPs and PdNPs bioreduced by BPLE was analysed for size and shape using HRTEM. PtNPs were found to be very small ranging between 1 to 2 nm (Figure 2A). The particles were monodispersed and uniformly distributed over the surface of the grid (Figure 2B). Agglomeration was not observed indicating the stability of the particles. PdNPs were spherical to irregular in shape (Figure 2C). The particles were very small ranging from 5to7 nm and well dispersed (Figure 2D). EDS spectra confirmed the presence of elemental platinum and palladium in the PtNPs and PdNPs, respectively (Figure 3A & 3B). Particle size distribution recorded using dynamic light scattering for PtNPs and PdNPs synthesized using BPLE was found to be well in agreement with the HRTEM results (Figure 4A & 4B).
FTIR analysis
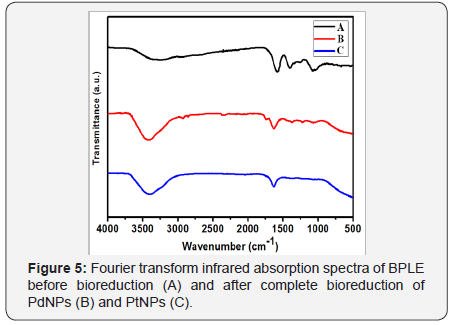
In order to understand the mechanism behind the bioreduction of the metal ions to their corresponding nanoparticles FTIR analysis was performed which showed that BPLE plays an important role. It reduced the metal salts to their respective nanoparticles and stabilizing them as well. This could be confirmed from the FTIR spectra of the BPLE before and after reduction of the nanoparticles. The FTIR spectra of the BPLE, before (Figure 5A) and after reduction of the salts Pd (Figure 5B) and Pt (Figure 5C), respectively, were recorded. Few characteristic peaks of the BPLE were noticed to be intact while few showed shift/disappearance, after the reduction of metal salts. This shift/disappearance can be due to the interaction of the corresponding functional group with the metal salt, and their role in stabilizing the nanoparticles. The broad peak at ~ 3276 cm-1 indicated the hydroxyl (-OH) group of polyphenols/alcohol. The peak at 1396 cm-1 diminished which represents (NO3-) or (symmetric CH3 bending of proteins), indicating their active role in reducing the metal salt. Similarly, the peak at 1249 cm-1 got reduced which represents C-S bond which might be utilized in nanoparticles formation.
Antiproliferative activity
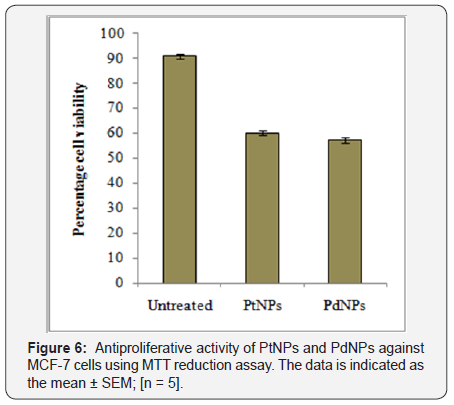
Both PtNPs and PdNPs synthesized by BPLE were checked for antiproliferative activity against MCF-7 cells using MTT dye reduction assay. 48hours of incubation exhibited significant reduction of the cell viability as compared to the untreated control. On treatment with PtNPs, MCF-7 cells showed 60.08±2.4 % viability while treatment with PdNPs showed 57.22±1.68 % viability (Figure 6). Hereby, PdNPs were found to be superior anticancer agents as compared to PtNPs as they efficiently inhibited the proliferation of MCF-7 cells.
Flow cytometric analysis
Flow cytometric analysis was carried on to evaluate the induction of apoptosis in presence of PtNPs and PdNPs synthesized by BPLE. Dual staining with annexin V-FITC and propidium iodide indicated externalization of phosphatidylserine and loss of cell membrane integrity, respectively. Compared to control MCF-7 cells treated with PtNPs after 48 hours showed 21.48 % of population were annexin V-FITC+PI+ indicating induction of apoptosis (Figure 7A & B). Similarly, the dual parametric dot plots combining annexin V-FITC and PI fluorescence in MCF- 7cells treated with PdNPs after 48hours showed 20.36% of population were annexin V-FITC+PI+ (Figure 7C). These results confirmed that the mechanism behind antiproliferative activity of the bio reduced PtNPs and PdNPs was apoptosis.

Confocal microscopy
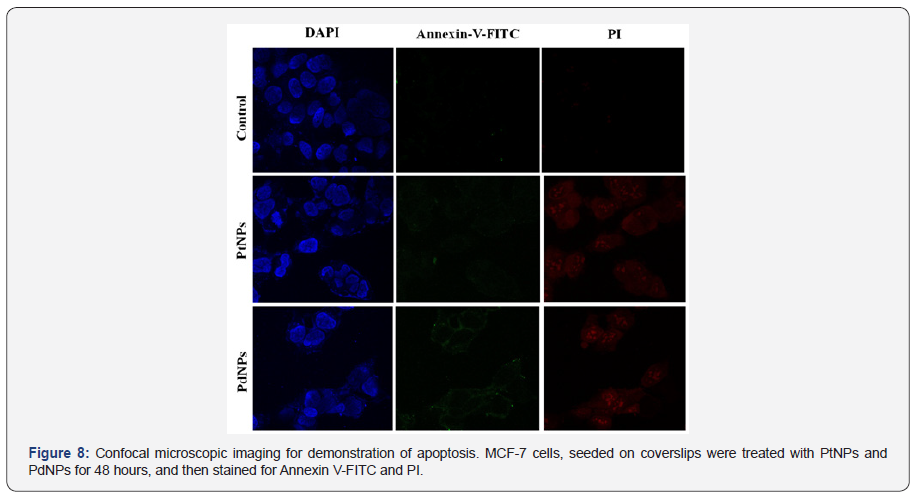
Mechanism behind the anticancer activity of bioreduced PtNPs and PdNPs against MCF-7cell lines was further confirmed using confocal microscopy (Figure 8). Confocal micrographs of the cells treated with PtNPs and PdNPs revealed valuable indications about induction of apoptosis. In case of the untreated control only DAPI was visible while on treatment with PtNPs annexin V-FITC+PI+ MCF-7 cells were visualized. However, the intensity of FITC was significantly low indicating slow induction of apoptosis. In case of PdNPs treated annexin V-FITC+PI+ MCF- 7 cells, the intensity of both FITC and PI were high indicating maximum cells were in late apoptotic stage.
Discussion
In an attempt to synthesize PtNPs and PdNPs using medicinal plants, we have demonstrated the nanobiotechnological potential of BPLE. The one pot synthesis was found to be rapid and ecofriendly. Prominent colour change and UV-visible spectral analysis confirmed that the synthesis of PtNPs was completed within 5 hours which was faster as compared to the synthesis by Fusarium oxysporum which took 8 hours for 90 % reduction of platinum salt [30]. High temperature of 100°C facilitated the reduction which was also observed for synthesis of PtNPs using Ocimum sanctum [31] Even synthesis of PdNPs using BPLE was faster as compared to Cinnamomum camphora leaf extract which took 48 hours, as reported earlier [32].
The absence of the absorption peaks above 300 nm indicated complete reduction of the initial Pd(II) ions which is consistent with the theoretical study of the surface plasmon resonance absorption of PdNPs. Moreover, broad absorption continua extending throughout the visible-near-ultraviolet region is typical of PdNPs colloid having a particle size of less than 10 nm [33-37] Increase of reaction rate with an increase in reaction temperature was reported in previous reports during synthesis of AuNPs and AgNPs using D. bulbifera and lemon grass [17,38]. In this study, we found that it took 5 hours to complete bioreduction of PtNPs and PdNPs at 100°C which was much slower than 20 minutes which was required for synthesis of AuNPs by G. glauca [19].
This relatively low rate of synthesis can probably be attributed to difficulty in initial stages of nuclei formation, which reflects in longer reaction time and higher temperature for achieving 100% conversion of metal salt to respective nanoparticles [39]. Our results are well in agreement with the previous report where PtNPs synthesized by Persimmon leaf broth were smaller in shape ranging from 2-20 nm and spherical in shape [39]. The average particle size of the PtNPs synthesized using BPLE were found to be between 1 to 2 nm which is very close to the PtNPs formed using D. kaki leaf broth (5 nm at 95°C). Higher temperature might play a key role in controlling size as it facilitates very rapid nuclei formation by most of the metal ions limiting the secondary reduction on the surface of preformed nuclei [23,40].
PdNPs synthesized by BPLE were also 5 to 7 nm in size similar to those formed using C. camphora leaf broth where the mean particle size was 6 nm [41]. Biogenic nanoparticles are primarily stabilized by metabolites like terpenoids that have functional groups of amines, alcohols, ketones, aldehydes, and carboxylic acids while reduction can be brought about by both reducing sugars and terpenoids. Similarly, ascorbic acid is a reducing agent that can reduce metal ions and form metal nanoparticles [31]. Likewise, citric acid is both reducing and stabilizing agent. Earlier, reports demonstrated that various components such as alkaloids, steroids, terpenoids, coumarins, flavones, hydroxybenzenes, anthracenes, lactones, reducing sugars, polysaccharides, amino acids, and proteins present in medicinal plants played a critical role in synthesis and capping of biogenic nanoparticles [32]. FTIR spectra indicated the functional groups from diverse phytochemical constituents in BPLE that might play a critical role in reduction and stabilization. Various alkaloid, flavonoids, saponins, tannin, steroid, terpenoids, sterol (stigmasterol), phenolic compound and essential oil are reported from B. prionitis leaf, the notable among which are glycosides (6-o-trans-p-coumaroyl-8-oacetylshanzhiside methyl ester, barlerinoside, shanzhiside methyl ester, 6-o-trans-p-coumaroyl- 8-o-acetylshanzhiside methyl ester, barlerin, acetylbarlerin, 7-methoxydiderroside, and lupulinoside), terpenoid (lupeol), pipataline, balarenone and 13,14-seco-stigmasta-5,14-diene-3- ol [25].
Previously, we have reported the presence of various groups of phytochemicals including ascorbic acid, citric acid, polyphenols, flavonoids, reducing sugars and starch in BPLE [21]. Thus the reduction of the metal salts to corresponding nanoparticles is highly rationalized by the diverse phytochemistry of BPLE. Bioreduced PtNPs and PdNPs exhibited potent anticancer activity against MCF-7 cells. PtNPs and complexes are reported to be superior anticancer agents against ovarian, liver, lung, head and neck, endometrial, bladder and oesophageal cancers. Similarly, cisplatin is also accepted as alternative option in therapies of several other solid tumors, including liver, gastric, brain, melanoma and soft-tissue sarcomas [41-43].
Likewise, PdNPs and organometallic palladium complexes are also reported as potential anticancer agents against blood, prostrate and cervical cancers [44,45].The mechanism of anticancer activity was found to be externalization of phosphatidyl serine and increase in membrane permeability which are considered to be the hallmarks of apoptosis [1,22,23]. Our earlier reports have demonstrated the promises of various metal nanoparticles synthesized by biological routes to be most biocompatible as well as bioactive showing multiple therapeutic potential like antimicrobial, antibiofilm, antituberculosis, antidiabetic, anticancer and antioxidant properties [46-56].The present report has provided a strong scientific rationale towards the use of phytogenic PtNPs and PdNPs in cancer drug discovery research.
Conclusion
This is the first report on synthesis of PtNPs and PdNPs using B. prionitis. The bioreduced nanoparticles were monodispersed, extremely small and stable which is a prerequisite for a candidate nanomedicine. The particles exhibited potent anticancer activity against human breast adenocarcinoma (MCF-7) cell lines. Treatment with PtNPs and PdNPs resulted in significant reduction in the viability of the cells. Dual staining method with annexin V-FITC and propidium iodide confirmed the mechanism of anticancer activity as apoptosis induction.
Acknowledgment
The authors acknowledge the help extended for the use of TEM and HRTEM facilities in Chemical Engineering and CRNTS funded by the DST through Nanomission and IRPHA schemes. We thank Mr. Tanay R. Bhagwat and Ms. Shriya Shende for drafting the research article. Dr. Geetanjali Tomar thanks DST INSPIRE for the faculty position and the research grant (IFA13 LSBM73 and GOI-E-161(2), respectively). The authors thank Dr. M. Jayakannan, Indian Institute of Science Education and Research (IISER), Pune for DLS facility.
References
- Ghosh S, More P, Derle A, Kitture R, Kale T, et al. (2015) Diosgenin functionalized iron oxide nanoparticles as novel nanomaterial against breast cancer. J Nanosci Nanotechnol 15(12): 9464-9472.
- Kitture R, Ghosh S, More PA, Date K, Gaware S, et al. (2015) Curcumin-loaded, self-assembled Aloe vera template for superior antioxidant activity and trans-membrane drug release. J Nanosci Nanotechnol 15(6): 4039-4045.
- Kitture R, Chordiya K, Gaware S, Ghosh S, More PA, et al. ( 2015) ZnO nanoparticles-red sandalwood conjugate: A promising anti-diabetic agent. J Nanosci Nanotechnol 15(6): 4046-4051.
- Kitture R, Ghosh S, Kulkarni P, Liu XL, Maity D, et al. (2012) Fe3O4- citrate-curcumin: Promising conjugates for superoxide scavenging, tumor suppression and cancer hyperthermia. J Appl Phys 3(6).
- Gaidhani SV, Yeshvekar RK, Shedbalkar UU, Bellare JH, Chopade BA (2014) Bio-reduction of hexachloroplatinic acid to platinum nanoparticles employing Acinetobacter calcoaceticus. Process Biochem 49(12): 2313-2319.
- Shedbalkar U, Singh R, Wadhwani S, Gaidhani S, Chopade BA (2014) Microbial synthesis of gold nanoparticles: Current status and future prospects. Adv Colloid Interface Sci 209: 40-48.
- Singh R, Nawale LU, Arkile M, Shedbalkar UU, Wadhwani SA, et al. (2015) Chemical and biological metal nanoparticles as antimycobacterial agents: A comparative study. Int J Antimicro Ag 46(2): 183-188.
- Wadhwani SA, Shedbalkar UU, Singh R, Karve MS, Chopade BA (2014) Novel polyhedral gold nanoparticles: green synthesis, optimization and characterization by environmental isolate of Acinetobacter sp. SW30. World J Microb Biot 30(10): 2723-2731.
- Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13(10):2638-2650.
- Adersh A, Ghosh S, More P, Chopade BA, Gandhi MN, et al. (2015) Surface defect rich ZnO quantum dots as antioxidant inhibiting α-amylase and α-glucosidase: A potential anti-diabetic nanomedicine. J Mater Chem B 3(22): 4597-4606.
- Ghosh S, Patil S, Ahire M, Kitture R, Kale S, et al. (2012) Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int J Nanomed7: 483-496.
- Ghosh S, Parihar VS, More P, Dhavale DD, Chopade BA (2015) Phytochemistry and therapeutic potential of medicinal plant: Dioscorea bulbifera. Med Chem 5(4):154-159.
- Ghosh S, Parihar VS, Dhavale DD, Chopade BA (2015) Commentary on therapeutic potential of Gnidia glauca: A novel medicinal plant. Med Chem 5(8):351-353.
- Ghosh S, Ahire M, Patil S, et al. (2012) Antidiabetic activity of Gnidia glauca and Dioscorea bulbifera: potent amylase and glucosidase inhibitors. Evid Based Complement Alternat Med 2012: 929051.
- Ghosh S, More P, Derle A, Patil AB, Markad P, et al. (2014) Diosgenin from Dioscorea bulbifera: Novel Hit for treatment of Type II Diabetes Mellitus with inhibitory activity against α-Amylase and α-Glucosidase. PLoS One 9(9): e106039.
- Ghosh S, Derle A, Ahire M, More P, Jagtap S, et al. (2013) Phytochemical analysis and free radical scavenging activity of medicinal plants Gnidia glauca and Dioscorea bulbifera. PLoS One 8(12): e82529.
- Ghosh S, Patil S, Ahire M, et al. Synthesis of gold nanoanisotrops using Dioscorea bulbifera tuber extract. J Nanomat 2011: 354793- 354800.
- Salunke GR, Ghosh S, Santosh Kumar RJ, Khade S, Vashisth P, et al. (2014) Rapid efficient synthesis and characterization of silver, gold, and bimetallic nanoparticles from the medicinal plant Plumbago zeylanica and their application in biofilm control. Int J Nanomedicine 9: 2635–2653.
- Ghosh S, Patil S, Ahire M, Kitture R, Gurav DD, et al. (2012) Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J Nanobiotechnology 10:17.
- Ghosh S, Gurav SP, Harke AN, Chacko MJ, Joshi KA, et al. (2016) Dioscorea oppositifolia mediated synthesis of gold and silver nanoparticles with catalytic activity. J Nanomed Nanotechnol 7: 398.
- Ghosh S, Chacko MJ, Harke AN, Gurav SP, Joshi KA, et al. (2016) Barleria prionitis leaf mediated synthesis of silver and gold nanocatalysts. J Nanomed Nanotechnol 7:394.
- Ghosh S, Harke AN, Chacko MJ, Gurav SP, Joshi KA, et al. (2016) Gloriosa superba mediated synthesis of silver and gold nanoparticles for anticancer applications. J Nanomed Nanotechnol 7:390.
- Ghosh S, Nitnavare R, Dewle A, Tomar GB, Chippalkatti R, et al. (2015)Novel platinum–palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: Anticancer and antioxidant activities. Int J Nanomedicine 10: 7477-7490.
- Ghosh S, Jagtap S, More P, Shete UJ, Maheshwari NO, et al. (2015) Dioscorea bulbifera mediated synthesis of novel AucoreAgshell nanoparticles with potent antibiofilm and antileishmanial activity. J Nanomater 2015:562938.
- Talukdar SN, Rahman MB, Paul S (2015) A Review on Barleria prionitis: Its pharmacognosy, phytochemicals and traditional use. J Adv Med Pharm Sci 4(4):1-13.
- Banerjee D, Maji AK, Mahapatra S, Banerji P (2012) Barleria prionitis Linn: A review of its traditional uses, phytochemistry, pharmacology and toxicity research. J Phytochem. 6(2): 31-41.
- Sontakke VA, Kate AN, Ghosh S, More P, Gonnade R, et al. (2015) Synthesis, DNA interaction and anticancer activity of 2-anthryl substituted benzimidazole derivatives. New J Chem 39(6):4882- 4890.
- Mallick A, More P, Ghosh S, Chippalkatti R, Chopade BA, et al. (2015) Dual drug conjugated nanoparticle for simultaneous targeting of mitochondria and nucleus in cancer cells. ACS Appl Mater Interfaces 7(14): 7584-7598.
- Pawar VU, Ghosh S, Chopade BA, Shinde VS (2010) Design and synthesis of harzialactone analogues: promising anticancer agents. Bioorg Med Chem Lett 20(24): 7243-7245.
- Govender Y, Riddin T, Gericke M, Whiteley CW (2009) Bioreduction of platinum salts into nanoparticles: a mechanistic perspective. Biotechnol Let 31(1): 95-100.
- Soundarrajan C, Sankari A, Dhandapani P, Maruthamuthu S, Ravichandran S et al. (2012) Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess Biosyst Eng 35(5): 827-833.
- Yang X, Li Q, Wang H, Jiale H, Liqin Lin, et al. (2010) Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf. J Nanopart Res 12(5):1589-1598.
- Yonezawa T, Imamura K, Kimizuka N (2001) Direct preparation and size control of palladium nanoparticle hydrosols by water-soluble isocyanide ligands. Langmuir 17(16): 4701-4703.
- Luo C, Zhang Y, Wang Y (2005) Palladium nanoparticles in poly(ethyleneglycol): the efficient and recyclable catalyst for Heck reaction. J Mol Catal A 229(1-2): 7-12.
- Ho PF, Chi KM (2004) Size-controlled synthesis of Pd nanoparticles from β-diketonato complexes of palladium. Nanotechnology 15(8): 1059-1064.
- Teranishi T, Miyake M (1998) Size control of palladium nanoparticles and their crystal structures. Chem Mater 10(2): 594-600.
- Nemamcha A, Rehspringer J, Khatmi D (2006) Synthesis of palladium nanoparticles by sonochemical reduction of palladium(II) nitrate in aqueous solution. J Phys Chem B 110(1): 383-387.
- Rai A, Singh A, Ahmad A, Sastry M (2006) Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir 22(2): 736-741.
- Song JY, Kwon EY, Kim BS (2010) Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst Eng 33(1):159-164.
- Song YJ, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32(1): 79-84.
- Hall MD, Amjadi S, Zhang M, Beale PJ, Hambley TW (2004) The mechanism of action of platinum (IV) complexes in ovarian cancer cell lines. J Inorg Biochem 98(10):1614-1624.
- Mohammadi H, Abedi A, Akbarzadeh A, Mohammad JM, Hasan E S et al. (2013) Evaluation of synthesized platinum nanoparticles on the MCF-7 and HepG-2 cancer cell lines. Int Nano Lett 3(1):28-32.
- Oberoi HS, Nukolova NV, Kabanov AV, Bronich TK (2013) Nanocarriers for delivery of platinum anticancer drugs. Adv Drug Deliv Rev 65(13-14):1667-1685.
- Carreira M, Calvo-Sanjuan R, Sanau M, Marzo I, Contel M (2012) Organometallic palladium complexes with a water-soluble iminophosphorane ligand as potential anticancer agents. Organometallics 31(16): 5772-5781.
- Kapdi R, Fairlamb IJS (2014) Anti-cancer palladium complexes: a focus on PdX2L2, palladacycles and related complexes. Chem Soc Rev 43(13): 4751-4777.
- Sant DG, Gujarathi TR, Harne SR, Ghosh S, Kitture R, et al. (2013) Adiantum philippense L. frond assisted rapid green synthesis of gold and silver nanoparticles. J Nanopart 2013: 1-9.
- Gaidhani, Sharvari S, Richa S, Divya Patel, Urvashi et al. (2013) Biofilm disruption activity of silver nanoparticles synthesized by Acinetobacter calcoaceticus PUCM 1005. Mater Lett 108: 324-327.
- Singh R, Wagh P, Wadhwani S, Gaidhani S, Kumbhar A et al. (2013) Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus’ and their enhanced antibacterial activity when combined with antibiotics. Int J Nanomedicine 8: 4277-4290.
- Wadhwani SA, Shedbalkar UU, Singh R, Chopade BA (2016) Biogenic selenium nanoparticles: Current status and future prospects. Appl Microbiol Biotechnol 100(6): 2555-2566.
- Singh R, Nawale L, Arkile M, Wadhwani S, Shedbalkar U, et al. (2016) Phytogenic silver, gold, and bimetallic nanoparticles as novel antitubercular agents. Int J Nanomedicine 11: 1889-1897.
- Singh R, Shedbalkar UU, Wadhwani SA, Chopade BA (2015) Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Appl Microbiol Biotechnol 99(11): 4579-4593.
- Singh R, Nadhe S, Wadhwani S, Shedbalkar U, Chopade BA (2016) Nanoparticles for control of biofilms of Acinetobacter species. Materials 9(5):383-399.
- Wadhwani SA, Shedbalkar UU, Singh R, Vashisth P, Pruthi V, et al. (2016) Kinetics of synthesis of gold nanoparticles by Acinetobacter sp. SW30 isolated from environment. Indian J Microbiol 56(4): 439- 444.
- Patil SS, Shedbalkar U, Truskewycz A, Chopade B A, Ball AS (2016) Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environmental Technology and Innovation 5:10-21.
- Pooja D, Sharvari G, Hitendra M, Yogesh S, Narhe R et al. (2015) Biosynthesis of gold nanoparticles by human microbiota from healthy skins. J Nanomed Nanotechnol 6:300.
- Ghosh S, More P, Nitnavare R, Jagtap S, Chippalkatti R, et al. (2015) Antidiabetic and antioxidant properties of copper nanoparticles synthesized by medicinal plant Dioscorea bulbifera. J Nanomed Nanotechnol S6: 007.






























