Synthesis and Characterization of Pure and Co doped Zinc oxide
Jyotsna Chauhan*, Varsha R Mehto and Shailendra Singh
HOD Nanotechnology, Rajeev Gandhi Technical University, India
Submission: January 30, 2017; Published: February 15, 2017
*Corresponding author: Jyotsna Chauhan, HOD Nanotechnology, Department of Physics, Rajeev Gandhi Technical University, India; Email: jyotsna chauhan2006@gmail.com
How to cite this article: Jyotsna C, Varsha R M, Shailendra S. Synthesis and Characterization of Pure and Co doped Zinc oxide. Glob J Nano. 2017; 1(2): 555558. DOI: 10.19080/GJN.2017.01.555558
Abstract
Present work is a study on synthesis and Characterization of pure ZnO and Co doped nanoparticles by using chemical co-precipitation method. We have doped pure ZnO with Co by 3% mole concentration. Structural and optical properties of these nanoparticles are analyzed by using XRD, UV-Visible spectroscopy. We found the crystallite size of range 20 nm to 30 nm.
Introduction
ZnO is a wide band gap (3.37 eV at room temperature) semiconductor of the II-VI semiconductor group. Nanosized particles of semiconductor materials have gained much more interest in recent years due to their desirable properties and applications in different areas such as catalysts [1] sensors [2] photoelectron devices [3,4] highly functional and effective devices [5]. Many methods have been used to synthesize ZnO nanoparticles such as sol - gel, electrochemical deposition, sonochemical, hydrothermal technique etc. In this work, we report the synthesis and characteristics of zinc oxide with Co nanoparticles obtained by using co-precipitation method. Synthesis and characterization of zinc oxide (ZnO) nanoparticles has found widespread interest during past few years due to their unique electro optical properties, which can be employed in devices such as ultraviolet (UV) light-emitting diodes (LEDs), blue luminescent devices, and UV lasers [6].
Experimental
Material to be used for synthesis the ZnO: Co nano particles are ZnSO4 .7H2O, CoSo4.7H2O (Cobalt sulfate), NaOH (Sodium Hydroxide), and DI (Distilled water). For keeping the size of particles in nano dimension the capping agent Ethileneglycol (EG) is used.
Result and Discussion
X-ray diffraction studies confirmed that the synthesized material was ZnO with wurtzite phase and the entire diffractionpeak agreed with the reported JCPDS data (card No. 36-1451). No characteristic peaks were observed other than ZnO. The X- ray diffraction data were recorded by using Kα radiation (1.5406 A°). The average grain size was calculated with the help of Scherrer’s equation using the FWHM of all peaks (Figure 1 & 2) show the XRD data for pure and Co doped ZnO nanoparticles.
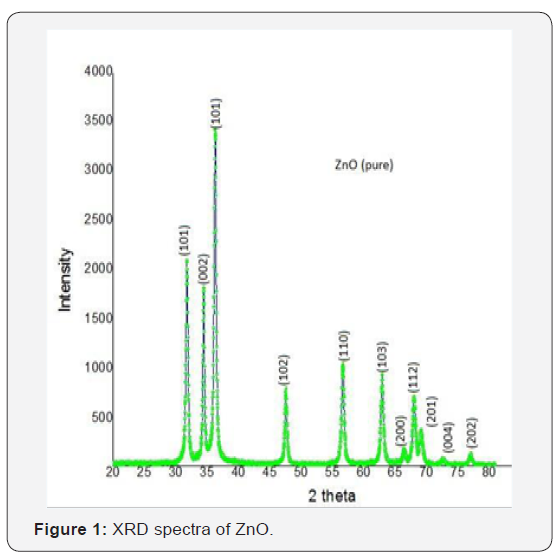
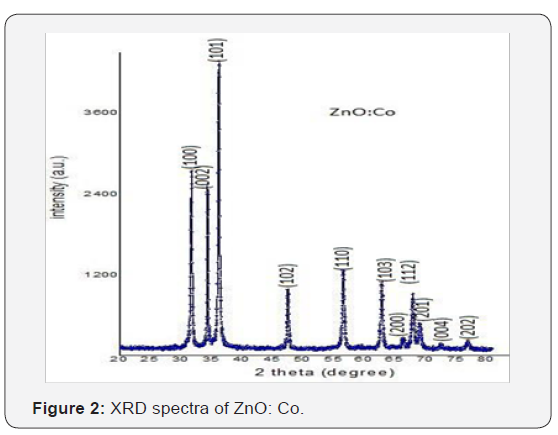
XRD
For calculating the particle size we used Scherer’s formula it is given by
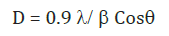
Where:
λ is a wavelength of x-ray (1.54 A0).
Β is a FWHM (full width and half maxima) By observing the above table it is cleared that the most prominent peak got for both samples at 101.
Average StrainAverage Strain
The average strain of quantum dots can be calculated by using Stokes-Wilson equation which is given below.
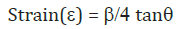
Where:
b is full width at half maxima (FWHM).
We have used the (101) reflexes of the x-ray diffraction profile to calculate the variations in the uniform strain as a function of ZnO and ZnO: Co nanoparticles and the results have been shown in figure.
Non Uniform Strain
It is denoted by (η). For study of non uniform strain changing with the particle size can be seen by using Hall equation which is given below.
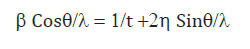
Where, ‘λ’ is the wavelength of x-ray used for scattering experiment and ‘t’ is the particle size and β is the line broadening.
UV-Visible Spectroscopy
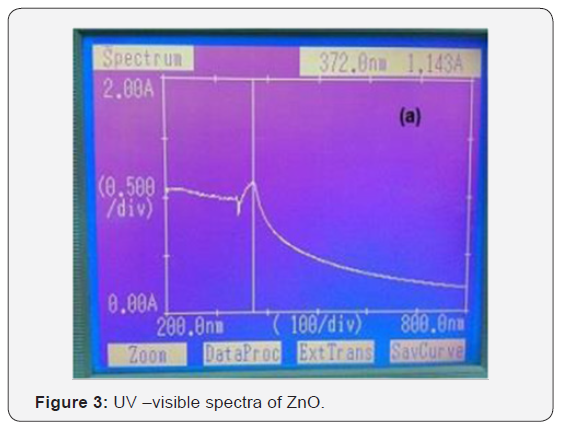
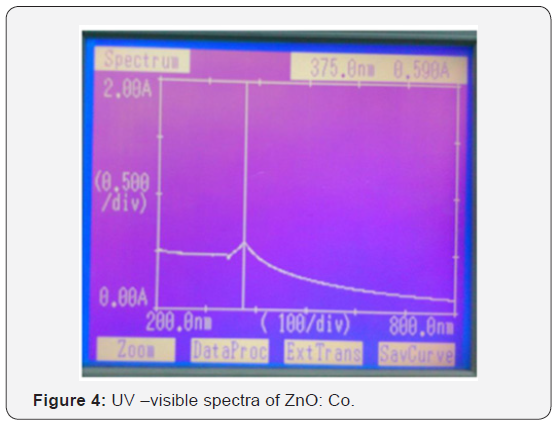
UV-spectroscopy is the technique which is used for finding the optical properties of material like band gap of material can be calculated by using this technique. But for calculating the band gap we use tauc equation [αhν = C (hν − Eg) 1/2]. Here αand C are the constant which depends on material. And Eg is the band gap of material and hν is the excited energy. The electronic states of spherical ZnO nanoparticles were carried out by UV– vis diffuse reflectance spectroscopy as shown in (Figure 3 & 4). The distinct sharp absorption at the band edge confirms that asprepared ZnO have a crystalline nature [7].
Acknowledgements
Both samples were prepared using co-precipitation method. The XRD analysis clearly indicates that pure phase of ZnO, and ZnO: Co is formed. Pure ZnO sample has the lowest grain size that is 22 nm, Co doped have 17nm respectively. Average strain is depends on the size of the particle as the size of the particle decrease strain is increases. Optical band gap is calculated using absorption spectra and it was found 2.65ev-3.02eV. Wavelength in UV-Vis spectra is shifted from lower to higher.
References
- Satyawati S Joshi, Prajakta R Patil, Madhav S Nimase, PP Bakare (2006) Role of ligands in the formation, phase stabilization, structural and magnetic properties of α-Fe2O3 nanoparticles. Journal of Nanoparticle Research 8(5): 635-643.
- Cheng XL, Zhao H, Huo LH, Gao S, Zhao (2004) JG: ZnO nanoparticulatethin film: preparation, characterization and gas-sensing properties. Sens Actuators B 102: 248-252.
- Sang Yeol Lee, Eun Sub Shim, Hong Seong Kang, Seong Sik Pang, Jeong Seok Kang (2005) Fabrication of ZnO thin film diode using laser annealing. Thin Solid Films 473(1): 31-34.
- Wang ZL, Kong XY, Ding Y, Gao P, Hughes WL, et al. (2004) Semiconducting and piezoelectric oxide nanostructures induced by polar surfaces. Advanced Functional Materials 14(10): 943-956.
- Huang YH, Zang Y, Liu L, Fan SS, Wei Y, et al. (2006) Controlled synthesis and field emission properties of ZnO nanostructures with different morphologies. J Nanosci Nanotechnol 6(3): 787-790.
- Chopra K L, Majora S, Pandya D K (1983) Transparent conductors-A status review. Thin Solid Films 102(1): 1-46.
- Lexi Zhang, Jianghong Zhao, Jianfeng Zheng, Li Li, Zhenping Zhu (2011) Shuttle-like ZnO nano/microrods: Facile synthesis, optical characterization and high formaldehyde sensing properties. Applied Surface Science 258(2): 711-718.






























