Exploring Soil Carbon and Nutrient Profiles in the Sundarbans Mangrove Forest of Bangladesh
Md. Sharif Hasan Limon, Md. Naif Ahmed Chowdhury, Shraboni Deb, Narottam Paul, Chameli Saha and Mahmood Hossain*
Forestry and Wood Technology Discipline, Khulna University, Khulna, Bangladesh
Submission: January 15, 2025;Published: January 24, 2025
*Corresponding author: Mahmood Hossain, Forestry and Wood Technology Discipline, Khulna University, Khulna, Bangladesh
How to cite this article: Md. Sharif Hasan L, Md. Naif Ahmed C, Shraboni D, Narottam P, Chameli S, et al. Exploring Soil Carbon and Nutrient Profiles in the Sundarbans Mangrove Forest of Bangladesh. Ecol Conserv Sci. 2025; 4(4): 555645.DOI:10.19080/ECOA.2025.04.555645
Abstract
Mangroves are globally significant ecosystems known for their carbon storage and nutrient cycling capabilities. Sundarbans is the largest contiguous mangrove forest in the world and is located in the south-west of Bangladesh. This study examines the carbon and nutrient (nitrogen, phosphorus, potassium) dynamics in the soil profile of the Sundarbans, the largest mangrove forest, focusing on pioneer and non-pioneer stands. Soil samples (n=150) from 30 sites were analysed for organic carbon and nutrients at 0-100cm depths. Results showed no significant (p>0.05) variation in carbon and nutrients (nitrogen, Phosphorus and Potassium) concentration across soil layers and stands. Significant (p<0.05) relationships between nutrient stocks and increasing depth were observed. The statistical models for the stock of carbon, nitrogen, phosphorus, and potassium were y = 1.0859*X + 39.184, R² = 0.65; y = 1.5455*X0.0669, R2=0.58; y = 0.0386x + 1.0606; R² = 0.89; and y = 1.1992x + 17.332; R² = 0.97 respectively. Within the top 1 m of soil, total stocks of carbon, nitrogen, phosphorus, and potassium were estimated at 212.21t/ha, 8.25t/ha, 5.88t/ha, and 104.65t/ha, respectively. These findings underscore the Sundarbans’ role in carbon sequestration and nutrient cycling, which is vital for mitigating climate risks and sustaining ecosystem functions. The study highlights the need for comprehensive assessments beyond 1 m depth to capture total ecosystem carbon and nutrient stocks accurately.
Keywords:Carbon; Mangroves; Nitrogen; Phosphorus; Potassium; Sundarbans
Introduction
Mangroves are important forest ecosystems in the tropical and subtropical sheltered intertidal zones, forming 15 million hectares of forests worldwide that provide habitat for rich biodiversity, ranging from bacteria, fungi, and algae to invertebrates, birds, and mammals [1]. It is one of the most productive ecosystems in terms of net primary productivity worldwide [2]. Mangroves can store much carbon deep in the soil profile [3]. Complex root structures, high sedimentation rates, waterlogged soils, and anoxic soils in mangroves influence C burial and higher turnover rate [2,4]. They can store carbon more rapidly than other ecosystems [5]. Mangroves occupy less than 1% of the continental surface area but have a major contribution to soil carbon storage globally [3]. Mangrove habitats stocked an average of 693t Corg ha−1 which is 15–24% of tropical coastal ocean carbon burial rate of 184g Corg m−2 yr−1 and is higher than the carbon stock in rain forests (241t C ha−1), peat swamps (408t C ha−1) salt marshes (593t C ha−1) and seagrasses (142.2t C ha−1) [6]. The growing recognition of mangroves as an efficient natural carbon sink is driving researchers to discover the state of soil carbon storage in the mangrove.
The variation of soil organic carbon (SOC) in a specific site largely depends on species composition [7] and stand age [8]. Mangrove plant species have high productivity due to the sink of organic C and the influence of available essential nutrients [2]. Nutrient content in the soils and their availability is one of the major factors influencing mangrove forest composition, structure, and productivity [9]. Nutrients availability varies spatially and temporarily in mangroves and also within a mangrove stand [9]. A variety of biotic and abiotic factors (tidal inundation, elevation in the tidal frame, soil type, nutrient input and output sources, redox status and microbial activities of soils, plant species, tree architecture, litter production, and decomposition) control the availability of nutrients to mangrove plants [2]. Considering the adaptive capacity of mangrove species with salinity, waterlogged conditions, luxury consumption of nutrients, and withstand cyclones, it is a well-suited plant community in the tropical and sub-tropical coastal zone. Nutrient uptake and nutrient-use efficiency vary with significant differences in species’ response to nutrient [4,10]. Therefore, assessing an ecosystem’s carbon and nutrient dynamics may help to understand the function of the ecosystem.
The Sundarbans is the world’s largest contiguous natural mangrove forest and one of the oldest managed mangrove forests in the world. A wide range of salinity (0.5-30 ppt) with a seasonal fluctuation might have influenced the hosting of more plant species in the Sundarbans [11]. The forest types of the Sundarbans are Heritiera, Excoecaria, Ceriops, Son-neratia, Heritiera–Excoecaria, Heritiera–Xylocarpus/Heritiera–Xylocarpus–Bruguiera, ExcoecariaCeriops, Excoecaria–Heritiera, Ceriops–Excoecaria, Xylocarpus–Bruguiera–Avicennia [12]. The Sundarban mangrove plays a pivotal role in soil carbon storage, which is important in mitigating climate risks [13]. So, carbon accounting in mangroves is important to reduce the impact of climate change. Recent studies have documented above-ground carbon stock in the Sundarbans [14-16]. Bangladesh Forest Department quantified carbon stock (Above and below-ground ground) during 2009- 2010 [17] and 2020 [13] in the Sundarbans of Bangladesh. Zaman et al. [18] evaluated carbon stock based on dominant species and provided comprehensive information about ecosystem carbon at the species level. However, assessing the soil carbon and nutrient dynamics at different depths along with vegetation types is vital for understanding ecosystem processes and guiding sustainable management practices.
Soil carbon is a key component of global carbon cycles, significantly influencing climate change mitigation through its sequestration potential [19]. The distribution of nutrients across soil depths varies due to plant uptake, microbial activity, and litter decomposition, with dis-tinct patterns evident under different vegetation types [20]. Moreover, vegetation influences soil properties by contributing organic matter through litterfall and root exudates, shaping nutrient cycling and microbial communities [21]. Depth-specific assessments are essential for identifying the storage potential of deep soil carbon and nutrients, which remains underexplored despite its importance in long-term carbon sequestration [22]. Therefore, this study aimed to assess the variation of carbon and nutrient content and stock at different depth ranges of soil up to 1 m depth in pioneer species-dominated stands and non-pioneer species-dominated stands of the Sundarbans. It is believed that the findings of this study provide more insights into carbon and nutrient cycling within Sundarbans mangrove systems, which support their productivity and resilience against environmental stressors such as salinity, flooding, and anthropogenic impacts. Moreover, the knowledge gained will help understand how different mangrove species influence soil properties and guide species selection for resto-ration projects, ensuring their ecological and functional sustainability.
Materials and Methods
Study site
The study was conducted in the Sundarbans, which lies between 21°27′30′′ and 22°30′30′′ N and 88°02′00′′ and 89°00′00′′ E, in the southwest of Bangladesh (Figure 1). The forest comprises of 4,120km2 of forest area and 1,897km2 of water body which represents the 6,017 km2 of Bangladesh part [23]. The rainfall pattern of the Sundarbans varies with the season and location. Maximum rainfall is observed in monsoon (80%) and the coast region (2790 mm) of the Sundarbans while 1800mm at Khulna region. Beside this, the temperature also varies with the seasons such as 26-34°C occur in the March to June and 12-25°C in December to February. The topography of Sundarbans is also different in forest and coastal area and the ground is about 0.9 to 2.11m elevated than the mean sea level. Silty clay loam soil with different layers makes the unique characteristics of the soils of Sundarbans where the annual relative humidity is about 70-80% with semi-diurnal tides (2-4m) [11, 24]. The Sundarbans is rich in both floral and faunal diversity, and it is the home to 22 families of plant species which represent 30 genera. Heritiera fomes, Excoecaria agallocha, Ceriops decandra, and Sonneratia apetala are the foremost plant species where Rhizophora mucronata, R.apiculata, Bruguiera gymnorrhiza, and Avicennia officinalis are also present with distinct pattern [25]. Depending on site quality, Sonneratia apetala and Avicennia officinalis are commonly found as pioneer tree species, and Heritiera fomes, Excoecaria agallocha, Ceriops decandra alone or in a mixture develop next seral community that are considered as non-pioneer.
Sample Collection
A total of 150 soil samples were collected from 30 sites from the Sundarbans using purposive stratified sampling representing different vegetation types (pioneer stand and non-pioneer stand). Three soil core (0 to 100cm) samples were collected from each site for determination of carbon concentration and nutrient concentration at the following depth (0 to 20cm, 20 to 40cm, 40 to 60cm, 60 to 80cm, and 80 to 100cm) by using open face (diameter 5cm) soil auger. The collected samples were then labelled and packed in an airtight plastic container for transportation to the laboratory for further analysis.
Sample processing
The collected soil samples were air dried at a temperature of about 30-35 ºC and samples were crushed, sieved through 2mm mesh and stored in air-tight plastic container according to Allen [26] for further analysis.
Determination of carbon and nutrients (N, P and K)
Organic carbon concentration in samples was determined by loss on ignition method [26]. Micro Kjeldahl digestion for Nitrogen and tri-acid (H2SO4, HClO4 and HNO3) digestion for Phosphorus and Potassium according to Allen [26]. Nitrogen and Phosphorus in the sample extract were measured calorimetrically according to the Baethgen & Alley [27] and Timothy et al. [28], respectively, using UV–visible Recording Spectrophotometer (HITACHI, U-2910, Japan). Potassium concentration in the sample’s extract was measured by Flame Photometer (PFP7, Jenway LTD, England).
Determination of carbon and nutrient stock
Bulk density of the corresponding layers and carbon and nutrients (N, P and K) stock in soil was estimated from their concentration of the respective layers. However, the bulk density of the different depths of the Sundarbans soil was taken from the database of Bangladesh Forest Department [13].
Statistical Analysis
The carbon and nutrients (N, P and K) in different layers of the pioneer species dominated species and non-pioneer were compared by two-way ANOVA analysis. The variation in carbon and nutrient concentration within different layers in a single and two different stands were evaluated by unpaired t-test using SAS statistical software. Different statistical equations (linear, logarithmic, exponential, and power) were tested to get best fit models for carbon and nutrients (N, P and K) stock in different layers (0-20cm, 20-40cm, 40-60cm, 60-80cm and 80-100cm) of soil profile. The level of significance adjusted R2 value, and the model types and trends were evaluated individually for carbon, nitrogen, phosphorus, and potassium using SAS (University edition) statistical software.
Results
Organic carbon
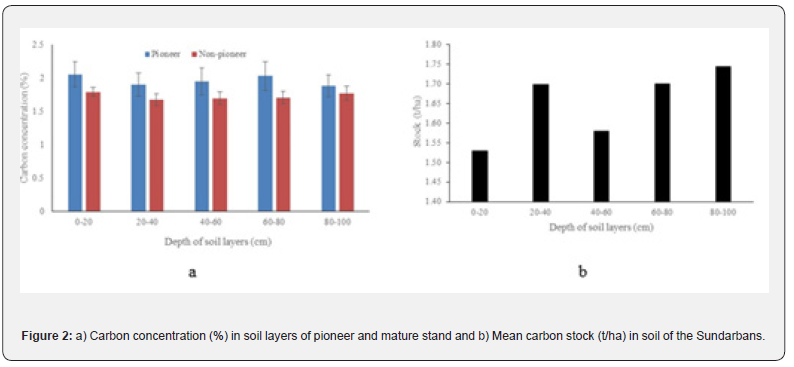
Carbon concentration in pioneer species dominated stand and non-pioneer species dominated stand ranged between 1.88- 2.06% and 1.67-1.79% respectively. While comparatively higher concentration (2.06%) was detected at the 0-20cm layer and lowest (1.88%) for the 80-100cm depth of pioneer stand. In case of non-pioneer stand, comparatively higher concentration (1.79%) was found at 0-20cm depth and lowest carbon concentration (1.67%) was found at 20-40cm layer. However, the organic carbon concentration did not vary significantly (p>0.05) among the different depths. (Figure 2a). Highest carbon stock (45.22t/ha) was observed for the depth of 60-80cm and lowest (40.38t/ha) was for 20-40cm depth. Total carbon stock for the depth of 1 m was 212.21t/ha (Figure 2b). The carbon stock showed significant linear positive relationship (y = 1.0859*X + 39.184; R² = 0.65, p<0.05) with the increasing the depth of soil profile.
Nitrogen
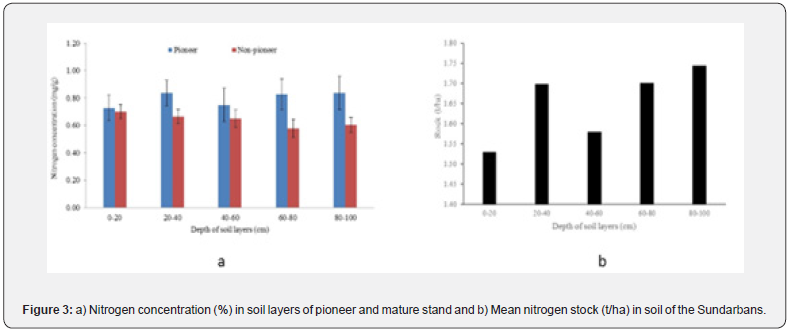
The ranges of mean concentration of total nitrogen for different depths of soil layers of pioneer and non-pioneer stands were 0.73-0.84mg/g to 0.58-0.70mg/g respectively. A comparatively higher nitrogen concentration (0.84mg/g) was detected in 20-40cm and 80-100cm depth of pioneer stand. For non-pioneer stand, a comparatively higher concentration (0.70mg/g) of nitrogen was observed for 0-20cm depth. The mean nitrogen concentration among the depths and stand types did not vary significantly (p>0.05) (Figure 3a). Comparatively higher stock (1.74t/ha) of nitrogen was detected at the lowest layer (80-100cm), while a lower amount (1.53t/ha) was observed for 0-20cm depth. However total nitrogen stocks up to 1m depth was 8.25t/ha (Figure 3b). While nitrogen stock at different depths of soil profile showed significantly powered positive relationship (y = 1.5455*X0.0669, R2=0.58, p<0.05) with the increasing depth of soil layers.
Phosphorus
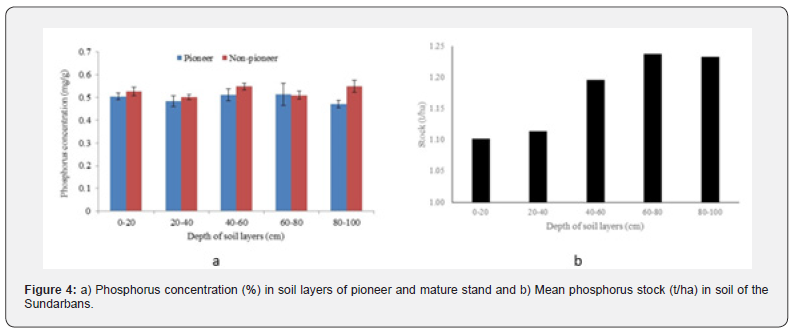
The mean total phosphorus concentration ranged between 0.47 to 0.51mg/g and to 0.51 to 0-0.55mg/g respectively for pioneer and non-pioneer stands at different depths of soil layers. Comparatively higher phosphorus concentration (0.51mg/g) was detected at 40 to 60 and 60-80cm depths of pioneer stand. A comparatively higher phosphorus concentration (0.55mg/g) was observed at 40-60 and 80-100cm depths. The mean phosphorus concentration among the depths and stand types did not vary significantly (p>0.05) (Figure 4a) in case of non-pioneer stand. A comparatively higher phosphorus stock (1.24 t/ha) was detected at 60-80 cm depth, while a lower (1.1t/ha) stock was observed at 0-20cm depth. However, total phosphorus stock was 5.88t/ha up to 1m depth (Figure 4b). Phosphorus stock showed a significant linear positive relationship (y = 0.0386x + 1.0606; R² = 0.89, p<0.05) with the increasing depth of soil profile.
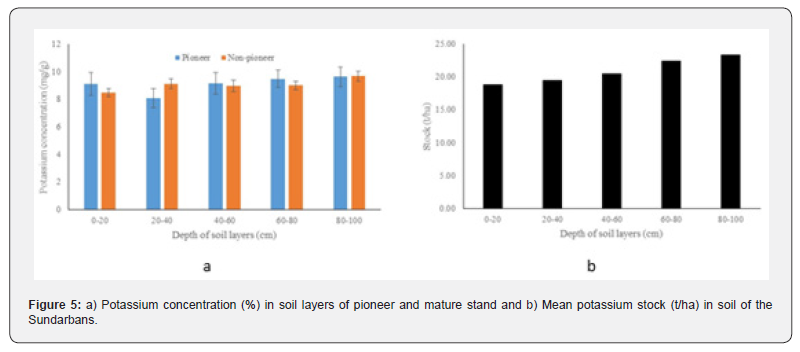
Potassium
The mean total potassium concentration of pioneer and non-pioneer stands ranged between 8.11 to 9.65mg/g and 8.99 to 9.68mg/g respectively at different depths of soil layers. A comparatively higher potassium concentration (9.65mg/g) was detected at 80-100cm depth in the pioneer stand. A comparatively higher potassium concentration (9.68mg/g) was observed at 80-100cm depth of the non-pioneer stand. The mean nitrogen concentration among the depths and stand types did not vary significantly (p>0.05) (Figure 5a). Higher potassium stock (23.39t/ha) was detected at the lowest layer (80-100cm), while a lowest stock (18.85t/ha) was observed at the topmost layer (0-20cm). However, the total stock of potassium up to 1m depth was 104.65t/ha (Figure 5b). Potassium stock showed a significant linear positive relationship (y = 1.1992x + 17.332; R² = 0.97, p<0.05) with the increasing depth of soil profile.
Discussion
Carbon is a fundamental element indicating the biological and physical health of the mangrove ecosystem. A significant fraction of their soil carbon is plant derived [29]. Soil organic carbon (SOC) in a mangrove ecosystem is affected by biotic and abiotic components and their functions [30]. Variation of soil organic carbon greatly depends on carbon source, mineral sediment [31], age of vegetation [32], disturbance history [33], dominant species [18], the depth of the organic soil, soil bulk-density, and salinity [34]. Complete inventories of ecosystem components show that carbon fixed within the forest and carbon imported from adjacent terrestrial and marine waters are stored as large pools of soil carbon [35]. The study revealed that organic carbon concentration did not vary significantly between pioneer and non-pioneer stand and soil depths. Uniform distribution of carbon content over depths was also reported by Suello et al. [36]. While significant variation in carbon stock was reported between old and young mangrove stands [36, 37]. Conversely, the structural component of mangrove stands, and their related functions were found to stabilize at the age of around 40 years, which is almost similar in dynamics to the natural stands [38]. The age of the studied pioneer-dominated, and non-pioneer species-dominated stands were above 40 years; this could be another reason not to find the variation in carbon content between the pioneer and non-pioneer stands of the Sundarbans. Brunskill [39] also mentioned slight variations or a small decline of organic carbon concentrations with sediment depth (up to 2m) in the mangrove soil. It is believed that soil physical characteristics, vegetation communities, and microbial activities significantly influence the carbon concentration and nature of mangrove soil [40, 41]. A comparatively higher carbon stock was detected at the deeper layers, while a lower amount was observed for 20-40 cm depth. The similar trend was observed by different authors [34]. The total carbon stock was 212.21t/ha, which is almost similar to 213.26t/ha that detected by GoB [13].
Nutrients are considered one of the most important parameters of the mangrove ecosystem influencing growth, reproduction and metabolic activities of biotic components. Nitrogen is one of the key essential elements for the sustenance of plant and animal life in the mangrove ecosystem [40]. Nitrogen fixation is generally low in mangrove sediments, diminishing the potential for nitrogen enrichment during decomposition [42], whereas the larger nutrient pools provide a potential for bacterial nitrogen incorporation directly from pore water pools [42]. Organic carbon and nutrients content in forest soil correlates positively [2]. This could be the reason to find similar pattern of carbon distribution in soil profile of the pioneer-dominated stands and non-pioneer dominated stands. A comparatively higher nitrogen stock at the deeper layers and a lower amount the surface layers, this variation may be related to the microbial immobilization, burial of organic matter, in the deeper layers of soil and higher tidal export of litter from the surface layer [18].
Phosphorus is one of the macro-nutrients which influence the plant physiology and may other aspects [43]. Soil texture plays an important role in nutrient retention. If the proportion of fine particles is higher in soil, then its nutrient retention capability becomes higher [44,45]. Being the Silty clay loam soil, mangrove sediment can trap significant amount of different nutrients. The Mangrove soil is a sink of high phosphorus quantity, and it is proved by the several studies that the undisturbed and dense mangrove forest contained higher amount of phosphorus which directed that the plant litter may be the source of the presence of higher amount of phosphorus in the soil [46].
Potassium is very leachable in saline conditions, and it could be the reason to detect lower concentration of potassium at the upper layers of soil. Conversely, higher potassium stock in the deeper layers is related to the higher concentration and higher bulk density of that layer. However, the variation in nutrient stock may be the result of soil characteristics of forest stands, sedimentation, bulk density, etc. among the soil layers and stand types [44]. In mangrove forests, the nutrients are supplied by the litterfall from trees and suspended materials from surface runoff above ground, fauna activities and microbial decomposition [47].
Conclusion
This study provides valuable insights into the carbon and nutrient distribution in the soil profiles of pioneer and non-pioneer stands within the Sundarbans mangrove forest. The apparent uniformity in the distribution of carbon, nitrogen, phosphorus, and potassium across soil depths and stand types highlights the influence of geomorphological settings, sedimentation patterns, and the age of the studied stands. Notably, the findings emphasize the carbon and nutrient stock of the Sundarbans ecosystem, with considerable observation within the top 1m of soil. This study cannot confirm 1m depth is enough for total ecosystem carbon and nutrient assessment in the Sundarbans. However, this study also underscores the need for further investigations beyond 1m depth to capture the complete picture of the Sundarbans’ ecosystem services. The results contribute to understanding the functional roles of mangrove forests in carbon storage and nutrient cycling, offering a basis for informed management and restoration practices. This knowledge is vital for enhancing the resilience and sustainability of mangrove ecosystems.
Acknowledgement
The study was funded by the Research and Innovation Centre, Khulna University. The authors are grateful for the field and lab facilities of Forestry and Wood Technology Discipline.
References
- FAO (2007) The world's mangroves 1980-2005. FAO Forestry Paper 153. Rome, Italy.
- Mahmood H (2004) Biomass, Litter Production and Selected Nutrients in Bruguiera Parviflora (Roxb.) Wight & Arn. Dominated Mangrove Forest Ecosystem at Kuala Selangor, Malaysia. PhD thesis, University Putra Malaysia, Seri Kembangan, unpublished.
- Bouillon S, Dahdouh-Guebas F, Rao AVVS, Koedam N, Dehairs F (2003) Sources of organic carbon in mangrove sediments: variability and possible ecological implications. Hydrobiologia 495: 33-39.
- Mahmood H (2014) Carbon pools and fluxes in Bruguiera parviflora dominated naturally growing mangrove forest of Peninsular Malaysia, Wetlands Ecology and Management 22(1): 15-23.
- Mackenzie R, Sharma S, Rovai AR (2021) Environmental drivers of blue carbon burial and soil carbon stocks in mangrove forests. In Dynamic sedimentary environments of mangrove coasts, pp. 275-294.
- Alongi DM (2022) Impacts of climate change on blue carbon stocks and fluxes in mangrove forests. Forests 13(2): 149.
- Ren H, Jian S, Lu H, Zhang Q (2008) Restoration of mangrove plantations and colonisation by native species in Leizhou bay, South China. Ecological Research 23: 401-407.
- Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M (2011) Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience 4(5): 293-297.
- Reef R, Feller IC, Lovelock CE (2010) Nutrition of mangroves. Tree Physiology 30(9): 1148-1160.
- Lovelock CE, Feller IC (2003) Photosynthetic performance and resource utilization of two mangrove species coexisting in a hypersaline scrub forest. Oecologia 134: 455-462.
- Siddiqi NA (2001) Mangrove Forestry in Bangladesh. Chittagong: Institute of Forestry & Environmental Science, University of Chittagong.
- Iftekhar MS, Saenger P (2008) Vegetation Dynamics in the Bangladesh Sundarbans Mangroves: A Review of Forest Inventories. Wetland Ecology and Management 16: 291-312.
- GoB (2019) Tree and forest resources of Bangladesh: The results of the Bangladesh Forest Inventory Cycle 1. Forest Department, Ministry of Environment, Forest and Climate Change, Government of the People’s Republic of Bangladesh, Dhaka, Bangladesh.
- Rahman MS, Donoghue, DN, Bracken LJ (2021) Is soil organic carbon underestimated in the largest mangrove forest ecosystems? Evidence from the Bangladesh Sundarbans. Catena 200: 105159.
- Kamruzzaman M, Ahmed S, Osawa A (2017) Biomass and net primary productivity of mangrove communities along the Oligohaline zone of Sundarbans, Bangladesh. Forest Ecosystems 4: 1-9.
- Kamruzzaman M, Ahmed S, Paul S, Rahman MM, Osawa A (2018) Stand structure and carbon storage in the oligohaline zone of the Sundarbans mangrove forest, Bangladesh. Forest Science and Technology 14 (1): 23-28.
- Rahman MM, Khan MNI, Hoque AF, Ahmed I (2015) Carbon stock in the Sundarbans mangrove forest: spatial variations in vegetation types and salinity zones. Wetlands Ecology and Management 23(2): 269-283.
- Zaman MR, Rahman MS, Ahmed S, Zuidema PA (2023) What drives carbon stocks in a mangrove forest? The role of stand structure, species diversity and functional traits. Estuarine, Coastal and Shelf Science 295: 108556.
- Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304 (5677): 1623-1627.
- Jobbágy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications 10(2): 423-436.
- Chapin FS, Matson PA, Mooney HA (2011) Principles of Terrestrial Ecosystem Ecology. Springer Science & Business Media.
- Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter-a key but poorly understood component of terrestrial C cycle. Plant and Soil 338(1): 143-158.
- Islam MS (2011) Biodiversity and livelihoods: a case study in Sundarbans reserve forest, world heritage and Ramsar site (Bangladesh). A master thesis, University of Klagenfurt, Austria.
- Gopal B, Chauhan M (2006) Biodiversity and its conservation in the Sundarbans Mangrove Ecosystem. Aquatic Sciences 68: 338-354.
- Mahmood H (2015) Handbook of selected plant species of the Sundarbans and the embankment ecosystem. Sustainable Development and Biodiversity Conservation in Coastal protection Forests, Bangladesh, GIZ GmbH, German Federal Ministry for Economic Cooperation and Development (BMZ).
- Allen SE (1974) Chemical analysis of ecological materials. Blackwell Scientific publication, Oxford.
- Baethgen WE, Alley MM (1989) A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjeldahl digests. Communications in Soil Science and Plant Analysis 20(9-10): 961-969.
- Timothy RP, Yoshiki M, Carol ML (1984) A manual of chemical & biological methods for seawater analysis. Pergamon Press, New York.
- Kristensen E, Bouillon S, Dittmar T, Marchand C (2008) Organic carbon dynamics in mangrove ecosystems: a review. Aquatic botany 89(2): 201-219.
- Hopkins WG, Huner NPA (2004) Introduction to Plant Physiology (3rd ed.). - John Wiley & Sons, Inc, Hoboken, NJ.
- Crooks S, Herr D, Tamelander J, Laffoley D, Vandever J (2011) Mitigating climate change through restoration and management of coastal wetlands and near-shore marine ecosystems: challenges and opportunities. Environment Department Paper 121, World Bank, Washington, DC
- Kridiborworn P, Chidthaisong A, Yuttitham M, Tripetchkul S (2012) Carbon sequestration by mangrove forest planted specifically for charcoal production in Yeesarn, Samut songkram. Sustainable Development of Energy, Water and Environment Systems 3: 87-92.
- Goodale CL, Apps MJ, Birdsey RA, Field CB, et al. (2002) Forest carbon sinks in the Northern Hemisphere. Ecological Applications 12: 891-899.
- Zou H, Li X, Li S, Xu Z, Yu Z, et al. (2023) Soil organic carbon stocks increased across the tide-induced salinity transect in restored mangrove region. Scientific Reports 13(1): 19758.
- Alongi DM, Sasekumar S, Chong VC, Trott LA, Tirendi F, et al. (2004) Sediment accumulation and organic material flux in a managed mangrove ecosystem: Estimates of land-ocean-atmosphere exchange in peninsular Malaysia. Marine Geology 208(2-4): 383-402.
- Suello RH, Hernandez SL, Bouillon S (2022) Mangrove sediment organic carbon storage and sources in relation to forest age and position along a deltaic salinity gradient. Biogeosciences 19(5): 1571-1585.
- Islam SM, Saha C, Mahmood H (2023) Biomass and carbon stocks in mangrove afforested areas, central coastal areas of Bangladesh. Environmental Challenges 13: 100784.
- Uddin MM, Hossain MM, Aziz AA, Lovelock CE (2022) Ecological development of mangrove plantations in the Bangladesh Delta. Forest Ecology and Management 517: 120269.
- Brunskill GJ, Zagorskis I, Pfitzner J (2002) Carbon burial rates in sediments and a carbon mass balance for the Herbert River region of the Great Barrier Reef continental shelf, North Queensland, Australia. Estuarine, Coastal and Shelf Science 54(4): 677-700.
- Alongi DM (2009) The Energetics of Mangrove Forests. Springer printed in USA.
- Matsui N, Meepol W, Chukwamdee J (2015) Soil organic carbon in mangrove ecosystems with different vegetation and sedimentological conditions. Journal of Marine Science and Engineering 3(4): 1404-1424.
- Kristensen E, Jensen MH, Banta GT, Hansen K, Holmer M (1998) Transformation and transport of inorganic nitrogen in sediments of a Southeast Asian mangrove forest. Aquatic Microbial Ecology 15: 165-175.
- Greger M (2004) Metal availability, uptake, transport and accumulation in plants. In: Prasad, M.N.V. (Ed.), Heavy Metal Stress in Plants -From Biomolecules to Ecosystems, (2nd edn). Springer-Verlag, Heidelberg, Berlin, Germany, pp: 1-27.
- Havlin JL, Tisdale SL, Nelson WL, Beaton JD (2014) Soil Fertility and Fertilizers: An Introduction to Nutrient Management. 8th ed. Pearson, Upper Saddle River, NJ.
- Nguyen H, Cao D, Schmitt K (2013) Soil particle-size composition and coastal erosion and accretion study in Soc Trang mangrove forests. Journal of Coastal Conservation 17(1): 93-104.
- Singh G, Chauhan R, Ranjan RK, Prasad MB, Ramanathan AL (2015) Phosphorus dynamics in mangroves of India. Current Science 108(10): 1874.
- Cannicci S, Burrows D, Fratini S, Smith TJ, Offenberg J (2008) Faunal impact on vegetation structure and ecosystem function in mangrove forests: a review. Aquatic Botany 89(2): 186-200.






























