Biosoap with Efficient Microorganisms to Reduce the Impact on Water Bodies
Scott Danniela1*, Waite Carolina1, de Sena André Pedral1, Baptista Neto José Antonio1 and da Silva Eduardo Camilo2
1Federal Fluminense University - DOT- Postgraduate Stricto Sensu Doctoral Student in Ocean and Earth Dynamics, Av. General Milton Tavares de Souza, s/o, 4th Floor , Red Beach Campus, Niterói, RJ, Brazil
2Federal Fluminense University, PPGAd /UFF - Postgraduate Program in Administration, Av. General Milton Tavares de Souza, s/n, 4th Floor, Campus da Praia Vermelha, Niterói, RJ, Brazil
Submission: May 15, 2024;Published: June 26, 2024
*Corresponding author: Scott Danniela, Federal Fluminense University - DOT- Postgraduate Stricto Sensu Doctoral Student in Ocean and Earth Dynamics, Av. General Milton Tavares de Souza, s/n, 4th Floor, Praia Vermelha Campus, Niterói, RJ, Brazil
How to cite this article: Scott Danniela*, Waite Carolina, de Sena André Pedral S, Baptista Neto José Antonio and da Silva Eduardo Camilo. Biosoap with Efficient Microorganisms to Reduce the Impact on Water Bodies. Ecol Conserv Sci. 2024; 4(3): 555638. DOI:10.19080/ECOA.2024.04.555638
Abstract
The development of more sustainable sanitizers is crucial to reducing the environmental impact of domestic effluents. Biosoap enriched with Effective Microorganisms (ME) presents itself as an ecological alternative to synthetic detergents. Comparatively, natural ingredients such as vegetable oils and fats demonstrate greater bio-degradability. In this study, it was observed that the initial Biosoap formulations achieved significant effectiveness in the parameters of conditioning, cleaning, bubble formation, persistence, hardness, solubility and drying. However, the viability of microorganisms in the initial formulations proved to be low, which led to the development of a microencapsulation technique to protect microorganisms during the saponification process. This technique, validated by previous studies, proved to be effective in preserving viable cells, facilitating the incorporation and action of ME, which resulted in improvements in the degradation processes of organic matter in simulated conditions of sanitary effluents. This study highlights the potential of microencapsulated Biosoap as a viable and efficient solution, encouraging more sustainable practices in waste management and effluent treatment.
Keywords: Biosoap; Effective Microorganisms (EM); Microencapsulation; Biodegradability; Wastewater treatment; Environmental sustainability; Saponification; Eco-friendly detergents
Abbreviations: EM: Effective Microorganisms; EPS: Extracellular Polymeric Substance; ABS: Alkylbenzene Sulfonate; BOD: Biochemical Oxygen Demands; COD: Chemical Oxygen Demands; PQ: Phred Quality
Introduction
The history of soap, according to some historians, it is believed that the discovery of the first soaps was accidental and the name is attributed to a Roman legend, around 1,000 BC in the vicinity of Morro do Sapo, in a small tributary of the River Tiber, below a religious site where animal sacrifice took place, washerwomen noticed that their clothes became cleaner when they came into contact with the soapy clay that dripped down the hill and into the water. The cleaning agent was formed by animal fat that mixed with wood ash and penetrated the clayey soil. It is believed that soap, close to the current one, originated with the Phoenicians 600 BC. Goat lard was boiled in water with wood ash, obtaining a creamy soap [1]. The most interesting thing is that the word “soap” is similar in several languages: Sapone (Italian), Savon (French), Seife (German), Saippua (Finnish), Szappan (Hungarian). With the First World War, there was a food shortage and consequently a lack of oils and animal fat for soap making and the solution was to use chemical additives such as surfactants [2]. With the change in habits towards the culture of bathing, skin treatments and the low cost that the chemical industry brought to the manufacture of soap, this led to an increase in the consumption of cleaning agents worldwide. And with increased consumption came the environmental impact of chemical additives and surfactants on water bodies. Since most chemicals are not biodegradable [3].
Surfactants can be synthesized chemically from petroleum derivatives or biotechnologically using microorganisms and renewable raw materials. Synthetic surfactants are more economically viable. However, when compared to bio-surfactants, they cause greater environmental impact. Therefore, biosurfactants are a promising alternative and their consumption is increasing every day [3]. Commercial detergents are based on synthetic surfactants produced chemically and manufactured from different raw materials, mainly petroleum derivatives. For example, alkylbenzene sulfonate (ABS), which is a synthetic detergent produced from benzene and propylene. The polluting potential of ABS is related to its ability to form a dense layer of white foam in bodies of water known as “detergent swan”, as occurs in the Tietê River along the cities of Santana do Parnaíba, Salto and Pirapora do Bom Jesus in the State of São Paulo, Brazil [2]. The dense layer of foam is responsible for carrying different types of pollutants over long distances, causing a decrease in the rate of photosynthesis, and altering the aquatic ecosystem [2].
Growing concern about the environmental impact of these products has led to the development of green chemistry, focused on minimizing the ecological footprint through the use of sustainable raw materials and environmentally friendly production processes. In this context, biosurfactants, which are secondary metabolites produced by microorganism such as Bacillus spp., have emerged as promising alternatives due to their superior biodegradability and lower toxicity [2]. Furthermore, biosurfactants have unique properties that include the ability to emulsify lipids and act as antibiotic agents, offering advantages in both industrial and environmental applications [4].
The present study aims to explore an innovation in soap production - Biosoap with the addition of Effective Microorganisms (EM) - which uses microencapsulation techniques to integrate and preserve these organisms during the saponification process. This approach not only improves the soap’s effectiveness in terms of cleaning and conditioning, but also enhances the biodegradation of organic compounds in effluents, aligning with regulatory requirements such as Brazilian Law No. 7,365 of 1985, which requires the inclusion of components biodegradable in cleaning products [5]. This work also aligns with global sustainability guidelines, emphasizing the need to reduce dependence on environmentally harmful synthetic chemicals. To reduce the impact of sanitary effluents on water bodies, studies began on microorganisms that were effective in degrading organic matter. The consortia contain microorganisms that will perform useful fermentative decomposition and microorganisms with physiological abilities to fix atmospheric nitrogen into amino acids and/or carbon dioxide into simple organic molecules through photosynthesis.
In known fermentation processes, Bioinsumo accelerates the breakdown of compounds such as Proteins, Sugars, Fats, Minerals and Fibers, promoting the rapid decomposition of organic matter. Furthermore, Bioinsumo still works in two primary ways:
a) By competitive exclusion of other undesirable Microorganisms that cause bad odors and environmental inefficiency (e.g. methanogenic bacteria, E. Coli, etc.).
b) By producing beneficial bioactive substances that promote environmental health, such as: enzymes, organic acids, amino acids, and antioxidants.
The genus Bacillus spp can be highlighted that is described and analyzed in its main mechanisms of action, such as the excretion of antibiotics, toxins, siderophores, lytic enzymes and induction of systemic resistance; focusing on its ability to be used as a biological control agent for pests and diseases in plants; as well as its use in the formulation of biopesticides, which were incorporated into agroecology programs; also used to treat various effluents and environmental accidents. It is a species of gram-positive bacteria that is a common saprophyte of soil and water, below are some studied for this research: Bacillus subtilis - Barros (2007), states that B. subtilis produces a biologically active compound called sur-factin, due to its great surface activity it is used in petroleum waste treatments, in addition to being extremely important in the industrial effluent treatment process.
i. Bacillus megaterium - Gomes (2003), states that B. megaterium has the capacity to produce biosurfactants and enzymatic expression for the degradation of aliphatic and aromatic compounds; showed promise for use as inoculum in bioremediation processes for areas contaminated with petrochemical waste, as they can use oily sludge as the only carbon source and produce biosurfactants.
ii. Bacillus licheniformis - In the study by Sanches [6], it was shown that B. licheniformis had a degradation capacity of 24% of the total concentration of the organochlorine pesticide Aldrin. Other factors, in the same study, such as exposure to sunlight and volatilization increased pesticide degradation to 31%.
iii. Bacillus polymyxa - is a gram-positive, mesophilic bacterium that produces oval spores, with thick walls, characterized by producing 2,3-butanediol and acetoin, also producing ethyl acetate, ethanol, and diacetyl, and is also a nitrogen fixer (LUERCE, 2002).
iv. Bacillus amyloliquefaciens - B. amyloliquefaciens IT-45 has a biochemical profile very similar to that of B. subtilis but has a higher molecular percentage of Guanine + Cytosine bases in its DNA and produces more α-amylase (LIMA, 2017); In the work carried out by Hlordzi (2020), where they simulated aquaculture effluent waters, it was possible to observe that B. amyloliquefaciens was efficient in removing nitrite, but its ammonia removal was dependent on the pH and water temperature conditions.
v. Bacillus cereus - has a biochemical profile very similar to that of B. megaterium; according to Wróbel [7] this bacterial genus has several bioremediation strategies, including biosorption, biosorption mediated by extracellular polymeric substance (EPS), bioaccumulation or bioprecipitation.
A good bioremediator, therefore, must be able to reduce the impact caused by pollutants present in sanitary effluents. Bacillus spp. can reduce the amounts of metals, such as: lead, cadmium, mercury, chromium, arsenic or nickel; In addition to metals, the Bacillus spp. They are also capable of reducing the amounts of BOD5,20 and COD, nitrogenous compounds, and phosphorus. The genus Bacillus spp, such as: Bacillus subtilis, Bacillus licheniformis, Bacillus amyloliquefa-ciens IT-45, Bacillus cereus, Bacillus coagulans and the genus Phenibacillus, such as Phenibacillus polymyxa, are good examples of bioremediators [7]. The objective of this research was to develop a methodology that would make it viable to introduce effective microorganisms into Biosoap, to accelerate the biodegradation of carbonaceous organic matter present in sanitary effluents represented by the Biochemical and Chemical Oxygen Demands (COD and BOD5,20) Nitrogen and Phosphorus, becoming a solution to reduce the environmental impacts caused by irregular releases.
Materials and Methods
To begin the research, it was necessary to develop a bar soap recipe that was biodegradable and easy to manufacture. To this end, two tests were carried out, the first known as the hot process, which consists of heating the vegetable oil until the temperature of the material reaches 70°C, adding stearic acid, stirring until it is well homogenized at a temperature around 70°C and 80°C, use a heated bath, at this point 73% sodium hydroxide at 99% purity is added, dissolved in warm water, to the mixture of vegetable oil and stearic acid, stirring until homogeneous, add 96% ethanol and complete the remainder of the recipe and stirring at constant speed, maintaining the temperature of the material at around 70° and 80°C until the ingredients are well homogenized and the mixture becomes transparent, after which the essence is added to the base and then poured into the molds to dry.
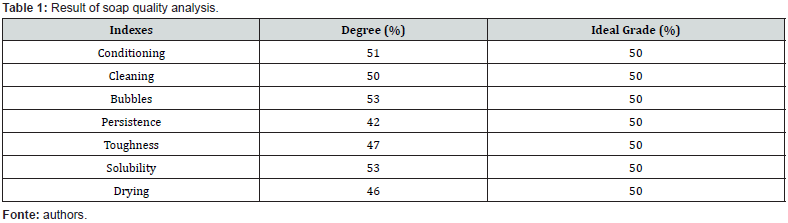
The second soap-making test was the cold process, where the heat from the lye results in a chemical reaction of the sodium hydroxide when it comes into contact with the vegetable oil, starting the saponification process. The bleach is prepared with 60% cold water and 30% sodium hydroxide at 99% purity, stirring until completely diluted, waiting to cool until the temperature reaches between 36° and 40°C. While the lye cools, prepare the vegetable fat base with 70% palm kernel oil, 30% soybean oil, add the coloring. After homogenizing the dye, add the bleach, always stirring at a constant speed of 17 rpm Figure 1 (c) and (d). Wait for the mixture to reach a temperature of 25°C and pour it into the molds to dry. It is worth mentioning that in the two recipes tested, 10% microorganisms were added (ME01 and ME02).
These tests aimed to evaluate the efficiency in the following indices: Conditioning; Cleaning; Bubbles; Persistence; Toughness; Solubility and Drying for Biosoap viability. To test the viability of microorganisms effective in Biosoap, initially two commercial blends (inoculum) of microorganisms (ME01 and ME02) were chosen. To this end, the ME01 product was activated considering the manufacturer’s recommendations on Bioaugmentation of colony forming units (CFU/100mL) in a quantity of 10^8 CFU/100mL. This process used 5% of the blend (inoculum), 5% of molasses (carbon source) as activator and 90% of clean, chlorinefree water (proportion 1:1:9) after using a mixer for 1h at 30 rpm, Figure 1 (a) and (b), which inoculum was left to grow for 5 days at room temperature.

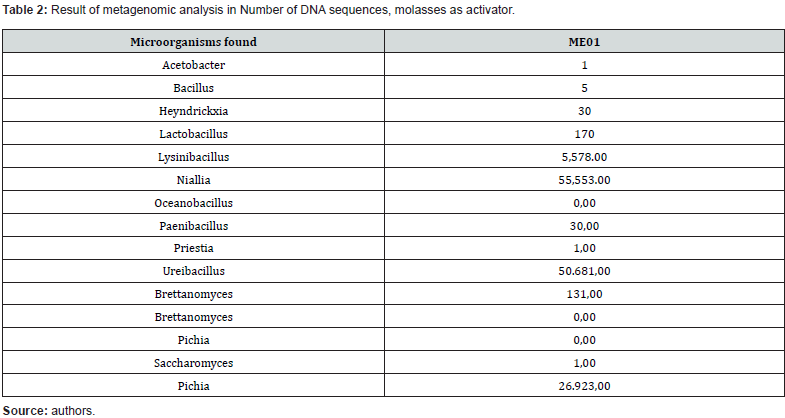
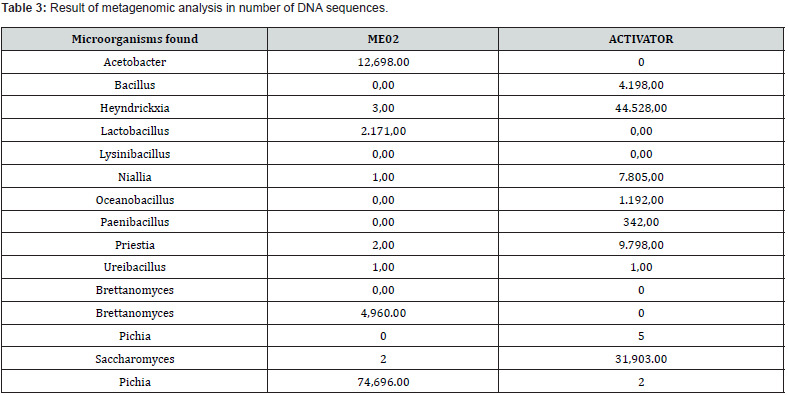
The ME02 product was activated, considering the manufacturer’s recommendations, with the Activator (carbon source) supplied together with the blend (inoculum). For bioaugmentation, 10% of the blend was used, 2% of the Activator (powder) dissolved in 90% of chlorine-free water, to homogenize the solution, a mixer was used for 1h at 30 rpm, Figure 1 (a) and (b), the inoculum was left to grow for 5 days at room temperature. After the growth period, aliquots were taken for metagenomics and for testing in Biosoap itself. For the Metagenomic analysis of bacteria, high-performance sequencing of the V3-V4 regions of the 16S rRNA gene was performed. Library preparation followed a proprietary protocol (Neoprospecta Microbiome Technologies, Brazil). Amplification was carried out using primers for the V3-V4 region of the 16S rRNA gene, 341F with sequence (CCTAC-GGGRSGCAGCAG), and 806R with sequence (GGACTACHVGGGTWTCTAAT), doi: 10.1371/journal. pone.0007401 / doi: 10.1038/ismej.2012.8). Libraries were sequenced using the MiSeq Sequencing System (ILLUMINA Inc., USA). For paired-end sequencing, V3 kits with 600 cycles or V2 kits with 500 cycles can be used. If single end, the V2 kit with 300 cycles is used.
The sequences were analyzed using the SENTINEL PIPELINE, where FASTA files are evaluated for Phred Quality (PQ) using the FastQC v.0.11.8 program (ANDREWS, 2010). Next, the FASTA files are subjected to trimming for low quality primers and sequences (Phred < 20). The proprietary software used for this purpose was built in Python v.3.6, which was inspired by the features of the BioPython project [8]. For PAIRED-END data, before the trim-ming step, two pairs of files (R1 and R2) are joined into a single file using pandaseq v.2.11 [9]. CLUSTERS with an abundance lower than 5 are removed from the analysis, as such structures are normally related to chimera sequences [10]. Taxonomic identifications are performed with blastn v.2.6.0+ [11], using a proprietary or public database as a reference. Regarding the definition of a species, among the 20 hits returned for each cluster, a Python instruction evaluates whether one of three requirements would be met by the HITS: 1) highest BIT-SCORE; 2) lower E-VALUE; and 3) taxonomies with greater representation. Species are defined using 99% identity.
The HITS that met one of the previous items were chosen as representative species. These analyzes were carried out on the Google Cloud computing platform, where Neoprospecta’s bioinformatics structure is hosted. DMD Bacterial and Fungal analyzes can be performed against reference databases for proprietary or public 16S rRNA and ITS genes. The public databases we had available are QUAST [12] and Greengenes [13]. After testing the viability of microorganisms in the Biosoap, with the two Bioinputs (ME01 and ME02), the taxonomic identification of the microorganisms resistant to the saponification process was carried out, these were isolated and identified through Biomerieux’s API test. After identifying the microorganisms, it was placed in a culture medium suitable for bioaugmentation for use in a new test at Biosoap.
For the purpose of bio-increasing the number of viable cells in the Biosoap, the unprotected resistant microorganism isolated from the bio input, and the encapsulated and microencapsulated microorganism were tested; for this purpose, a commercial product (B. subtilis CCT 0089) was purchased. To carry out the experiment in the manufacture of Biosoap with these microorganisms (resistant and encapsulated and microencapsulated), the soap recipe (cold process) was used, as described above, after which it was placed in drying molds and 10% of the resistant microorganism was added with 1x108 UFC (liquid) and 1% of microorganisms encapsulated with 1x109 UFC (solid), whose capsule composition contained sodium chloride, Kaolin, silicon dioxide. They were left to dry for 24 hours, were unmolded, and cut, placed in boxes to rest for 6 weeks to finish the saponification process.
Results
The results of the soap tests showed that both recipes had low solidification, Figure 1, this was due to the low quality of the sodium hydroxide at 50% purity. To obtain better quality, it was decided to change the sodium hydroxide from 50% to 99% purity. With the change of more pure sodium hydroxide in the recipes, performance improved in the cold process, with the quality indexes in Table 1. With the biodegradable soap recipe stabilized, Figure 2(a), research began to inoculate with effective microorganisms from commercial blends. The research was focused on the preservation of viable cells of microorganisms, and the first tests carried out were with the commercial blend ME01, in which metagenomics was carried out to identify the microorganisms present in the product, Table 2. As the preservation of viable cells of microorganisms in the first tests was low and, in some cases, even null. Tests were started by varying the concentrations of the ME01 blend, changing the bioaugmentation method, application point and temperature control in the Biosoap manufacturing process. However, the results have not yet been satisfactory. Therefore, tests began with another commercial blend, ME02, in which metagenomics was also carried out to identify the microorganisms present in the product, Table 3.
Tests with ME02 followed the same manufacturing procedures as Biosoap with ME01. But the results were not satisfactory either. During Biosoap tests with blends ME01 and ME02 (inoculum), a low number of viable cells was observed, around 102 CFU. (Figure 3) were preserved. Being identified as the group Bacillus spp. in biosoap, although the growth was not significant, they managed to survive the saponification process. Taxonomic identification, through Biomerieux’s API test, of the isolated resistant microorganism indicated that it was Bacillus subtilis, then a bioaugmentation process began in culture medium until a concentration of 1x108 UFC was reached, Figure 3. A new test was carried out using the isolates in Biosoap but maintained the procedures with the manufacture of Biosoap from previous blends, Figure 4. But the results did not have a desirable concentration for good efficiency in the treatment of organic matter present in sanitary effluents. With the low concentration of viable cells in the experiments, studies were aimed at protecting the microorganisms and making them more resistant to the saponification process. The test began with encapsulated and microencapsulated microorganisms, whose capsule composition contained sodium chloride, kaolin, and silicon dioxide. And the con-centration of microorganisms, according to the manufacturer, was 1.0x108 UFC.

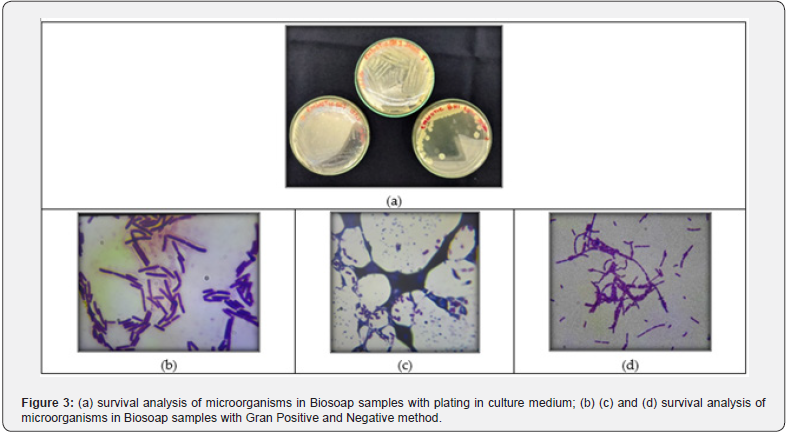



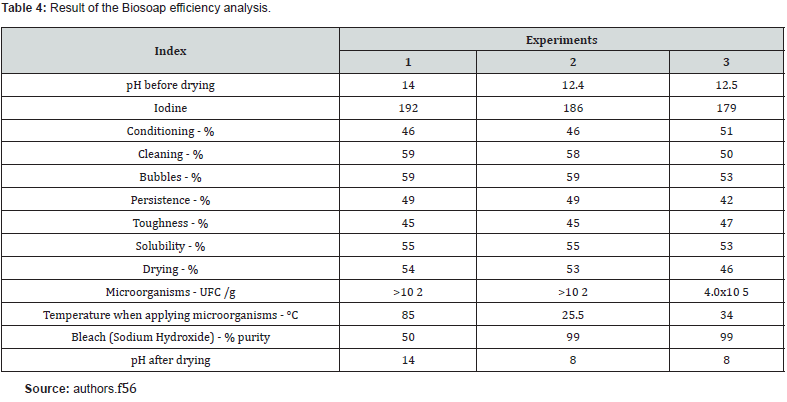
In the test with the encapsulated products, the procedures for manufacturing Biosoap from previous blends were maintained. However, the encapsulated material settled to the bottom of the mold (Figure 5), causing a lack of homogenization of the microorganisms throughout the Biosoap. So, the procedure for applying the encapsulated substances changed, and they were even added after the Biosoap mass went into shape. In this procedure, post-use survival reached 4.0x105 UFC /g with application of 1% of encapsulated microorganisms. A new test was carried out with a microencapsulated product, the result was satisfactory in visual appearance (Figure 6). Biosoap presented, in analysis, good quality in terms of cleaning agent parameters and mainly the concentration of viable cells (Table 4) for inoculation of the treatment system of a single-family residence.
Discussion
This study underscores the applicability and environmental benefits of Biosoap with Effective Microorganisms (EM), using microencapsulation techniques to improve the viability and effectiveness of microorganisms during the saponification process. The microencapsulation technique, already well established in the food and pharmaceutical industries to protect and release active ingredients in a controlled manner, has proven to be crucial for the stability of MEs under adverse conditions and during the saponification reaction [14,15]. Integrating effective microorganisms into cleaning products not only enhances the degradation of organic materials in wastewater, but also promotes in situ bioremediation, an emerging approach to wastewater treatment. Studies have demonstrated the ability of microorganisms such as Bacillus spp. in reducing the concentration of heavy metals and other pollutants, highlighting their potential role in mitigating the effects of aquatic pollution and improving water quality [7].
In the context of environmental legislation, products such as Biosoap respond directly to the demands for greener and sustainable solutions in daily consumption products. Brazil’s law nº 7,365 of 1985 is an example of how regulations can encourage innovation towards environmentally friendly products, requiring the incorporation of biodegradable components in detergents and soaps [16]. Furthermore, the discussion about the environmental impacts of synthetic detergents versus biological alternatives highlights a critical need for change in the industry. Biosoap represents a significant step in this direction, offering a practical and efficient alternative that combines effective cleaning with environmental responsibility.
For future research, it is recommended to explore a wider range of microorganisms and encapsulation techniques. Diversification of EM could offer a wider range of biological activities and increase product efficacy under different environmental conditions. Investigating the long-term impact of using Biosoap in real communities, especially in sensitive areas such as coastal or densely populated urban regions, would provide valuable insights into its effectiveness and environmental sustainability. Additionally, studies that evaluate the complete life cycle of Biosoap, from production to disposal, would help to better understand its general environmental impact and further optimize its formulation and application. This holistic approach is essential to meeting both consumer needs and environmental conservation imperatives.
Conclusion
The research demonstrated that it is highly viable for the manufacture of a sanitizing product using the cold process, where inoculation with micro-encapsulated microorganisms not only provided a favorable cost-benefit due to process efficiency and reduced energy consumption, but also resulted in a product with superior visual appearance and im-proved security and usability features. Furthermore, the use of microencapsulated effective microorganisms contributed significantly to the stability and prolonged effectiveness of the product, making it a sustainable and efficient alternative to conventional detergents. Considering the proven capabilities of Bacillus species as effective bioremediation agents in reducing heavy metals and reducing BOD5,20 and COD levels, as well as in the transformation of nitrogenous compounds and phosphorus, the Biosoap formulation was optimized to include a blend of specially selected microorganisms. The proposed composition includes Bacillus subtilis, Bacillus licheniformis, Bacillus amyloliquefaciens and Bacillus megaterium, each contributing 1.5x108 UFC/g, complemented by Saccharomyces cerevisiae with the same concentration, to maximize bioremediation efficacy. This strategic approach not only improves the product’s functionality as a cleaning agent, but also amplifies its role in mitigating adverse environmental impacts associated with the treatment and disposal of domestic wastewater.
References
- Dantas Filho, Francisco Ferreira, Silva GN Costa AS (2017) Teaching-learning process of the concepts of acids and bases with the inclusion of experimentation using the theme of ecological soap. Holos 2: 161-173.
- Felipe, Lorena de Oliveira, Sandra de Cássia Dias (2017) Synthetic surfactants and biosurfactants: advantages and disadvantages. Química Nova - São Paulo-SP, BR 39(3): 228-236.
- Hasan Fariha, Shah Aamer Ali, Hameed Abdul (2006) Aplicações industriais de lipases microbianas. Tecnologia enzimática e microbiana 39(2): 235-251.
- Martins Silvia, Biral Paula (2019) Natural Saboaria, cold saponification, Cold Process, Bahia, Brazil ©
- Anvisa (2008) Guide to quality control of cosmetic products - An approach to physical and chemical tests. Brasília p. 18 - 121.
- Sanchez D, Jose G, Henry L, Charles A (2012) Degradation of Aldrin by Bacillus licheniformis, isolated from water and sediments of Ciénaga Grande, Santa Marta, Colombia. Acta biol. Columbus 17(1): 67-76.
- Wrobel Monika, Wojciech Ś, Paweł K, Karol K, Jakub D (2023) Bioremediation of Heavy Metals by the Genus Bacillus. Int J Environ Res Public Health 20(6): 4964.
- Cock Peter JA, Tiago A, Jeffrey TC, Brad AC, Cymon JC, et al. (2009) BioPython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25(11): 14-22.
- Masella André P, Andrea KB, Jakub MT, Daniel GB, Josh DN (2012) PANDA seq: paired assembler for lighting sequences. BMC Bioinformatics 13: 1-7.
- Phipson Belinda, Smyth Gordon K (2010) Permutation P-values should never be zero: calculating exact P-values when permutations are drawn at random. Statist Appl Genet Mol Biol 9(39).
- Altschul Stephen F, Lipma David J (1990) Protein database searches multiple alignments. Ann Natl Acad Sci 87(14): 5509-5513.
- Gurevich A, Vladislav S, Nikolay V, Glenn T (2013) QUAST: quality assessment tool for genome assemblies. Bioinformatics 29(8): 1072-1075.
- Desantis Todd Z, Hugenholtz P, Larsen N, Rojas M, Brodie EL, et al. (2006) Greengenes, a chimera-verified 16S rRNA gene database and ARB-compatible workbench. Appl Environ Microbiol 72(7): 5069-5072.
- Laurenti Elisa, Garcia Sandra (2013) Efficiency of natural and commercial encapsulating materials in controlled release of encapsulated probiotics. Brazil J Food Technol 16: 107-115.
- Peanparkdee Methavee, Iwamoto Satoshi, Yamauchi Ryo (2016) Microencapsulation: a review of applications in the food and pharmaceutical industries. Rev Agri Sci 4: 56-65.
- Anvisa (1999) Resolution No. 482, of September 23, 1999, Technical regulation for establishing the identity and quality of vegetable oils and fats, Official Gazette of the Federative Republic of Brazil, Brasília.
- Baker IJA, Drummond CJ, Furlong DNN, Grieser F (2004) Surfactant bio-degradation: sugar-based surfactants compared to other surfactants. In: Zoller U (ed). CRC Press, 2004. Handbook of Detergents - Part B: Environmental Impact. Ch. 28: 739-760.
- Chimello CM, Bruza FB, Ramos MJ, Silva RCS, Kremer CK (2012) Study on the choice of the type of detergent used by con-sumers in Itatiba. Online Environmental Sciences Magazine 8(1): 60-61.
- Ghanbarzadeh M, Golmoradizadeh A, Homaei A (2018) Carrageenans and carrageenases: Versatile poly-saccharides and promising marine enzymes. Phytochem Rev 17(3): 535-571.
- Hlordzi Vivian, Felix KKA, Gyamfua A, Emmanuel DA, Yishan Lu, et al. (2020) The use of Bacillus species in maintenance of water quality in aquaculture: A Review. Aquacul Rep 18: 100503.
- Machado Thaís Strieder (2018) Production of bacterial biosurfactants in soils using bioaugmentation and biostimulation.
- Melo Itamar Soares de (2008) Environmental microbiology. ed. rev. amp. - Jaguariúna: Embrapa Meio Ambiente, pp. 647.
- Mulligan CN, Sharma SK, Mudhoo A, Makhijani K (2014) Green chemistry and biosurfactant research. In: Mulligan CN, Sharma SK, Mudhoo A (ed.). Biosurfactants: Res Trend Appl Ch 1: 1-30.
- Oliveira Hugo Mendes de (2021) Reduction of organic matter with application of Bacillus subtilis and B. licheniformis in sediments from a polyculture nursery of Litopenaeus vannamei with Oreochromis niloticus.
- Osadebe AU, Chinelo AO, Bukola MS, Gideon CO (2018) Microbial Degradation of Anionic Surfactants from Laundry Detergents Commonly Discharged into a Riverine Ecosystem. Department of Microbiology, University of Port Harcourt, Choba, Nigeria. J Appl Life Sci Int 16(4).
- Penteado JCP, EL Seoud OA, Carvalho LRF (2006) Linear alkylbenzene sulfonate: an environmental and analytical approach. New Chem 29(5): 1038-1046.
- Steemers Frank J, Gunderson Kevin L (2007) Whole Genome Genotyping Technologies on the BeadArray™ Journal of Biotechnology: Healthcare Nutrition Technol 2(1): 41-49.
- Favaro-Trindade, Carmen S (2016) Encapsulation of active/bioactive/probiotic agents. In: Edible Films and Coatings. CRC Press pp. 363-378.






























