Evaluation of Antimicrobial Activity of Cinnamon, Cinnamomum Cassia Plant Extract and Its Complementary Effects of The Plant Extracts and the Antibiotics
Safa Mustafa Ibrahim*, Mutaman Ali A Kehail and Elnour Elamin Abdelrahman
Department of Microbiology, Faculty of Sciences, University of Gezira, Wad-Medani, Sudan
Submission: November 17, 2023;Published: December 13, 2023
*Corresponding author: Safa Mustafa Ibrahim, Department of Microbiology, Faculty of Sciences, University of Gezira, Wad-Medani, Sudan. Email id: abuelhadi@hotmail.com
How to cite this article: Safa M I, Mutaman Ali A K and Elnour E A. Evaluation of Antimicrobial Activity of Cinnamon, Cinnamomum Cassia Plant Extract and Its Complementary Effects of The Plant Extracts and the Antibiotics. Ecol Conserv Sci. 2023; 3(5): 555625. DOI:10.19080/ECOA.2023.03.555625
Abstract
The current research endeavor aims to assess the antibacterial efficacy of Cinnamon cassia (C. cassia) and its potential synergistic effects when combined with antibiotics. The microdilution method was used to determine the minimum inhibitory concentration (MIC) of plant extracts against the tested bacteria. The disk diffusion method was utilized to assess the synergistic effect that plants and antibiotic extractions have on one another. According to the findings, the Cinnamon plant possesses antibacterial effects against the bacteria that were tested, albeit to varying degrees; this could be related to the active compounds found in the plant. In addition, the zones of inhibition that occurred from the combination of plant extract and antibiotics were larger than those that resulted from antibiotics used by themselves. This was the case for all the microorganisms that were examined. The methanolic extract of the plant was shown to have a greater inhibitory impact than the water extract of the plant did, as demonstrated by the results. It is recommended that further research be conducted on the extract of the plant to separate and identify the active elements.
Keywords: Disc Diffusion; Microdilution Method; Antibiotics Activity Assay; Secondary Metabolites; Synergistic Effects
Abbreviations:MIC: Minimum Inhibitory Concentration; BHI: Brain Heart Infusion; AS: Antibiotic-Sensitive; AI: Antibiotic-Intermediate; AR: Antibiotic-Resistant; MHA: Muller Hinton Agar
Introduction
Various components of medicinal plants are utilized in the extraction of raw medicines and exhibit diverse therapeutic qualities. Rural inhabitants acquired knowledge through a process of trial and error, wherein they developed the ability to differentiate between plants with advantageous properties. This was achieved by employing suitable combinations and processing techniques, so attaining consistent and optimal outcomes. Since ancient times, people have utilized medicinal plants as medications, and many of the herbs and spices they use to season food also contain beneficial medical ingredients. Herbal remedies are virtually always used to cure illness in non-industrialized countries. By the close of the 20th century, a few customs had come to dominate the field of herbal medicine. Many of the medications that are currently available to doctors were once used as herbal treatments, such as quinine, digitalis, opium, and aspirin. 80 percent of the world’s population currently uses herbal medicine for some part of primary health care [1-5]. Cinnamomum cassia (C. cassia) is a member of the Lauraceae family within the plant kingdom. The substance in question is obtained through the extraction process of the tree’s bark and is commonly employed as a flavor enhancer in diverse Asian culinary practices. The countries that commonly engage in cultivation include India, China, Uganda, Vietnam, Bangladesh, and Pakistan. The herb exhibits a strong aromatic profile and possesses a flavor that combines sweetness with a little hint of bitterness. The plant is additionally utilized in many modalities of traditional medicine.
In recent times, a multitude of research has documented the therapeutic use of this botanical specimen in many pathological states, including diabetes mellitus, peptic ulcer disorders, and other malignancies. In order to provide a thorough and current body of information about the pharmacological and therapeutic applications of this particular herb, a review was conducted on papers published during the past decade (2002-2012). According to Gernot [6], C. cassia has exhibited antibacterial properties against many illnesses. The antibacterial efficacy of the ethanol extract obtained from Cinnamomum cassia has been observed to be substantial in inhibiting the growth of Pseudomonas aeruginosa. The disc diffusion method was used to identify the antibacterial activity of cinnamon against wound pathogens such as Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae. Cinnamon has been found to possess significant antibacterial properties due to their ability to reduce the virulence and pathogenicity of drug-resistant bacteria in vivo. The rise in the occurrence of antibiotic resistance observed in clinical settings has been ascribed to various factors, including the improper utilization and excessive administration of antibiotics [7,8]. The present study aimed to investigate the antibacterial efficacy of Cinnamon cassia (C. cassia) and its potential synergistic effects when combined with selected antibiotics.
Materials and Methods
Collection of the Plant and Microorganisms Samples
The plant materials utilized in this research included of Cinnamon, which was procured from several locations inside the Wad-medani local market. The bacterial strains included in this investigation were Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumonia), Acinetobacter baumannii (A. baumannii), Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus), and Salmonella spp., all of which exhibited resistance to antibiotics. The bacteria were sourced from the microbiology laboratory of the Department of Biology, Faculty of Science, University of Hail, as well as from King Khalid Hospital, Hail. The bacteria were cultured on Brain Heart Infusion (BHI) agar medium (HiMedia) at a temperature of 4 ºC to prepare for subsequent investigations.
Preparation of Plant Extract
In the process of aqueous extraction, a quantity of 20 grams of air-dried powder was introduced into 150 milliliters of distilled water, and subsequently subjected to a gentle boiling process over a duration of 2 hours. Subsequently, the solution underwent filtration using eight layers of muslin cloth and was subjected to centrifugation at a force of 5000 times the acceleration due to gravity for a duration of 10 minutes. The resulting liquid portion, known as the supernatant, was carefully collected. The procedure was replicated on two occasions. Following a duration of 6 hours, the supernatant was gathered at regular intervals of 2 hours, consolidated, and subsequently subjected to concentration techniques, resulting in a final volume equivalent to one-fourth of the initial volume.
The Process of Preparing Culture Media, Inoculums, Reagents, and Antibiotics
The media types utilized in this investigation encompassed Brain Heart Infusion broth, Nutrient agar (bio-life), and Mueller- Hinton agar (HiMedia). The extraction method involved the utilization of methanol and water. The media and solvent were acquired from a commercial entity located in the city of Jeddah. The antibiotics utilized in the study, namely Vancomycin, Tetracyclines, Chloramphenicol, and Ampicillin, were procured from pharmacies located in Hail city. The procedure for the preparation of plant extracts at standard concentrations. The aqueous of each aqueous extract and alcohol pre-prepared (each separately) were utilized for the experiment. The aqueous extract was dissolved in 5 grams of sterile distilled water, whereas the alcoholic extract was diluted in 5 ml of Di Methyl Sulphoxide (DMSO). A standard concentration of aqueous and methanol extracts was obtained, resulting in a stock solution of 200 mg/mL. The aqueous extracts underwent sterilization, while the methanol extract was subjected to pasteurization at a temperature of 62 ºC for a duration of 15 minutes.
The Process of Preparing Inocula
The stock cultures of bacteria were preserved at a temperature of 4°C on nutrient agar slants. To generate active cultures for experimental purposes, a loopful of the desired culture will be transferred into a 5 ml volume of Brain Heart Infusion broth. The resulting mixture will then be incubated at a temperature of 37 °C for a duration of 24 hours.
Antibiotics Activity Assay
A Mueller-Hinton agar plate that had been contaminated with test bacteria was covered with antibiotic discs. A concentration gradient is produced during incubation as the antibiotics diffuse outward from the discs. The zone diameter of inhibition was assessed after 18 to 24 hours, and reference tables were used to identify whether the bacteria were antibiotic-sensitive (S), antibiotic-intermediate (I), or antibiotic-resistant (R).
Plant Extracts Activity Assay
According to Kumar et al. [9], the plant antibacterial activity was evaluated using the paper disk diffusion assay. On Muller Hinton Agar (MHA) medium, a suspension of the testing microorganisms was applied. After the agar plates are inoculated with the examined microorganisms, 20μl of plant extract (concentration 200 mg/ml) will be impregnated into the filter paper discs (5mm in diameter). After that, the plates were incubated for 24 hours at 37°C. Following incubation, the growth inhibition zone was measured by taking a millimeter reading of the zone of inhibition’s diameter.
MIC of Plant Extract Ascertained using the Microdilution Method
To prepare the 96-well plates, 50μl of Mueller-Hinton broth for bacteria were dispensed into each well. In the first row of the plate, 50μl was added from the stock solution containing tested extracts at a concentration of 200 mg/ml. Next, two-fold; a micropipette will be used to carry out the serial dilutions. Ten microliters of inocula were added to each well based on the obtained concentration range, which was between 100 and 0.1953 mg/ml. However, a positive control inoculum was adjusted to contain roughly 1.5X108 CFU/mL. An inoculum combined with medium served as the negative control, and plant extract was utilized as the positive control. For eighteen hours, the test plates were incubated at 37 °C. The plates were incubated for an additional hour after 18 hours, at which time 50 μl of a 0.01% solution of 2, 3, 5-triphenyl tetrazolium chloride (TTC) was added to the wells. The solution in the well remained clear following TTC incubation, indicating that the biological active bacteria had inhibited growth by reducing the colorless tetrazolium salt to a red-colored product. The lowest sample concentration (MIC) that demonstrated full growth inhibition and no color change (clear) was defined [10,11].
Complementary Effects of the Plant Extracts and the Antibiotics
For determination of the complementary relationship between antibiotics and plant extracts, at temperature of 37 °C, the bacterial cultures were cultured in BHI broth. Each bacterium was put onto the surface of Mueller-Hinton agar plates after it had grown for a period of four hours. After that, an antibiotic disk with a diameter of 5 mm was placed on the surface of each inoculation plate, and then 20 l of plant extract was added. This was done to determine whether there was a synergistic impact between the plant extract (with a concentration of 200 mg/ml) and antibiotics. The plates were kept in an incubator at 37 °C for a whole day. We shall take measurements of the diameters of the cleared zones.
Results and Discussion
Cinnamon Plant’s Antibacterial Properties
The largest pharmacies, plants produce endless combinations of bioactive ingredients that have an impact on both human and animal health [12]. They have a lot of secondary metabolites and could be used to make drugs. The current study investigated how the examined bacteria grew in response to methanol and Cinnamon water extracts. (Table 2) displays the tested bacteria’s inhibition zone widths in relation to the tested plant. Salmonella spp. and examined bacteria including E. coli, K. pneumonia, A. baumannii, P. aeruginosa, and S. aureus in this investigation. It is evident from the data in (Table 1) and (Figure 1) that most of the examined bacteria did not have their development inhibited by Cinnamon water extracts, except for P. aeruginosa and A. baumannii bacteria, for which the inhibition zone diameter was 2 mm. However, all studied bacteria were inhibited by the plant’s methanol extract to differing degrees; the inhibition zones for Salmonella spp. and E. coli, K. pneumonia, A. baumannii, P. aeruginosa, and S. aureus were 8, 2, 3, 5, and 4 mm, respectively. In general, the methanol extract of the medicinal plants that were researched demonstrates a larger inhibitory effect against the bacteria that were tested than the water extract did.
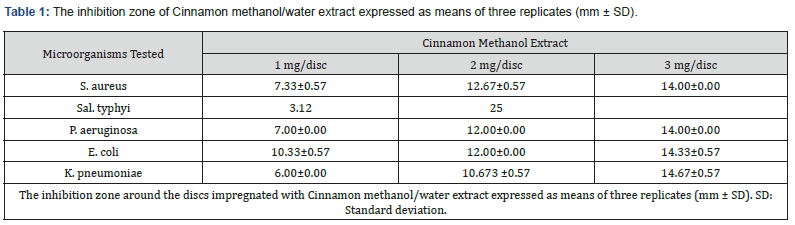
Antibacterial Activity of Cinnamon Methanolic/Water Extract
The results of the antimicrobial activity of Cinnamon methanolic extract are presented in (Table 1). The Cinnamon extract showed antibacterial activity in vitro against the tested microorganisms. The mean inhibition zone at the higher concentration of the extract (3 mg/disc) was 14.00±0.00mm, 14.00±0.00mm, 14.00±0.00mm, 14.33±0.57mm and 14.67±0.57mm, respectively. In contrast, the mean inhibition zone at the lower concentration of the extract (1 mg/disc) was 7.33±0.57mm, 7.00±0.00mm, 10.33±0.57 mm and 6.00±0.00mm, respectively. As indicated in (Table 1), the highly inhibited bacteria by the plant extract were K. pneumoniae.
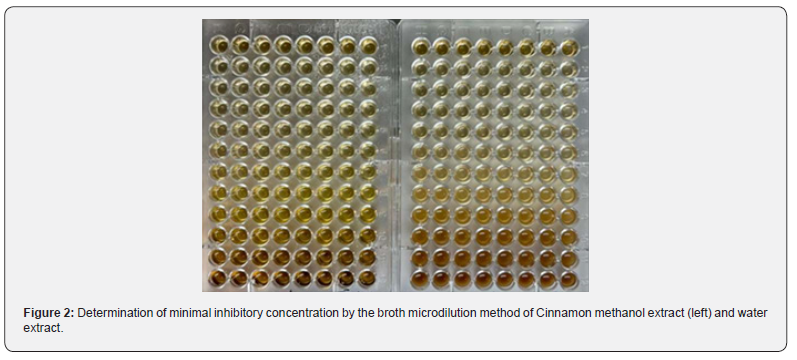
Minimum Inhibitory Concentration (MIC) of Cinnamon Plant Extract
The results of determining M.I.C.s and M.B.C.s values of the tested methanol and water extract of Cinnamon against the selective microorganisms were reported in (Tables 2, 3) and (Figure 2). The results showed that there was a reduction in the minimum inhibitory concentration (MIC) of the Cinnamon plant’s water extract against P. aeruginosa (6.25 mg/ml). The water extract and the methanolic extract of the plant both had MIC values of 50 mg/ml against E. coli, however the water extract had a higher value than the methanolic extract. In contrast, the minimum inhibitory concentrations (MICs) of water extract and methanolic extract against S. aureus, K. pneumonia, E. coli, and A. baumannii were 50 and 25, 12.25 and 5.125, 25 and 12.5, 12.5 and 12.5, and 12.5 and 6.25 mg/ml, respectively. Nkuo- Akenji et al. [13] used methanol extracts of plant parts commonly used in Cameroon for the treatment of typhoid fever. They tested the extracts for antibacterial activity against Salmonella typhi, S. paratyphi, and S. typhimurium. The researchers discovered that plant extracts with low MIC (1 mg/ml and lower) may contain compounds with therapeutic activity. The M.I.C. s values (Table 2) of Cinnamon water extract ranged from 2.16 to 6.25 mg/mL for the bacteria tested. M.B.C.’s values vary from 25 to 50 mg/ mL. Using the scheme proposed by Gatsing and colleagues [14], Cinnamon extract exhibited a bacteriostatic profile against the MRSA strain (MBC/MIC˂4). While the same extract exhibited bactericidal action against K. pneumoniae, P. aeruginosa, and E. coli (MBC/MIC ratio≥4).
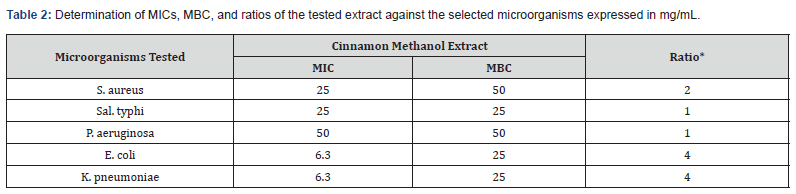
*MBC/MIC ratio are interpreted by using the scheme proposed by Gatsing [14].
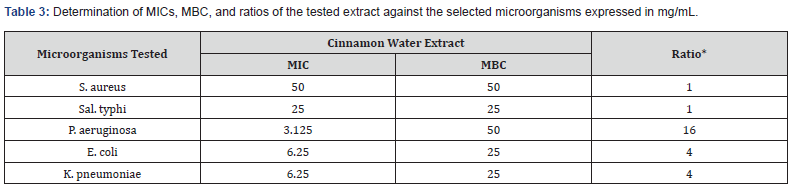
*MBC/MIC ratio, and MFC/MIC ratio are interpreted by using the scheme proposed by Gatsing [14].
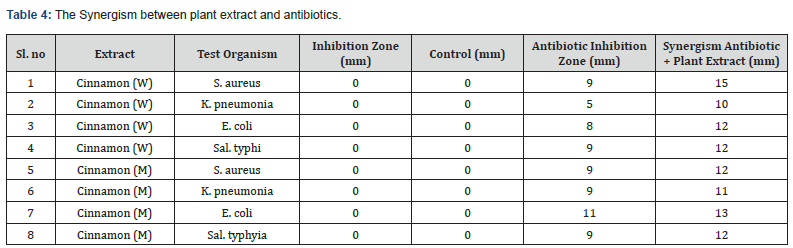
Synergism between plant extract and antibiotics
Table 4 illustrate the synergistic relationship that exists between Cinnamon plant extract and certain antibiotics. Antibiotics were added to the plant extracts. It was discovered that the plant extracts were able to block the growth of the microorganisms that were put to the test by utilizing antibiotics mixture. In addition, the diameters of the inhibition zones increased when the plant extracts were mixed with antibiotics mixture. Furthermore, the inhibition zones grew more prominent as more antibiotics were added. This indicates that the synergistic effect of antibiotics combined with plant extracts resulted in the suppression of tested bacteria to varied degrees. Nevertheless, the Cinnamon plant demonstrated higher inhibition. In addition, the effectiveness of the methanol extract was superior to that of the water extracts for all the plants examined. In general, the synergy between extracts of Cinnamon was the most efficient in inhibiting antibiotic-resistant bacteria The most inhibited bacteria were staphylococci aureus, with an inhibition zone diameter of 16 millimeters for the combination of Cinnamon and antibiotic and 9 millimeters for antibiotic solo. Combined with antibiotics, the water and methanolic extract of Cinnamon were very effective at inhibiting the growth of E. coli bacteria. The diameter of the inhibition zone was 12 millimeters for Cinnamon. In addition, the methanolic extracts of the plants tested with added antibiotics were significantly more effective than the water extracts of the plants tested with additional antibiotics. Cinnamon water and methanolic extract both could prevent the growth of Sal. typhi; however, combining the methanolic plant extract with an antibiotic was more successful.
Discussion
Several traditional medicinal uses for Sudanese medicinal plants have been documented. These include treating various microbial infections, wounds, cancer, gastrointestinal disorders, malaria, diabetes, rheumatic pain, respiratory system disorders, jaundice, and urinary system inflammations. Most of the pharmacological research supported conventional applications. Furthermore, several bioactive substances are active ingredients, including flavonoids, saponins, alkaloids, steroids, terpenes, tannins, fatty acids, and essential oils [9]. According to Ahmed and Mirghani [15], Ahmed et al. [16], Khalid et al. [17], and other researchers, Sudan’s poisonous plants (Elghazali et al., 2008) and sporadic scientific research studies on medicinal and aromatic plants have a legitimate place in health care as herbal pharmaceutically produced medicine or nutraceutical and dietary supplements in the nation’s economy and trade as for export, culinary purpose, as spice, condiments, fruits, and vegetables. The objectives of the present study were to assess the antibacterial and antioxidant properties of Cinnamon (Cinnamomum cassia), as well as their potential synergistic effects with antibiotic medications from Wad-Medani area, Gezira State, Sudan. Among the microorganisms under test were harmful bacteria. It was also assessed how well plant extract and antibiotic worked together.
There have been several reports of the antibacterial properties of various herbs and spices (Alicia, 1981) [5,7]. About 100 medicinal plants that are used by traditional healers in Rwanda to treat illnesses were evaluated for antibacterial qualities by Vlietinek et al. (1999). According to their research, 16 percent of the examined plants were active against Pseudomonas aeruginosa, 2% against E. coli, and 45% against Staph aureus. Demetrio et al. [18] looked at the antibacterial properties of crude ethanol extracts from 12 medicinal plants found in the Philippines. Psidium guajava, Phyllanthus niruri, Ehretia microphylla, and P. beetle ethanol extracts demonstrated the most potential against drug-resistant Gram-positive and Gram-negative bacteria. Abdalla and Abdallah [19] found that 142 Sudanese plant species from 64 families have antibacterial activities when extracted with polar and non-polar solvents and tested in vitro against gram positive and gram negative. The prevention of antibiotic resistance is an urgent matter that must be addressed by increased antimicrobial utilization as well as a reduction in hospital-acquired infections. Despite this, there is a pressing need to persist with the research and development of new antibiotic pharmaceuticals because these medicines are of the utmost significance in preserving the efficacy of antimicrobial treatment. The World Health Organization (WHO) estimates that in underdeveloped nations, around 75% of the population depends on plant-based remedies as an important requirement for human vital medicinal services [20]. These plant-based remedies are utilized as a component of their traditional therapeutic framework.
Antibiotic resistance is a problem that continues to affect the healthcare sector in a major part of the world in both developing countries and developed countries, and the development of multi-drug resistant bacteria in hospital settings is still a challenging problem [21]. In addition, the spread of antibiotic-resistant bacteria in hospitals is also a problem. The results of this investigation indicate that it may be possible to use these extracts in the treatment of bacterial disorders. The results of this study were encouraging, despite the need for clinical studies to determine the true sufficiency of the treatment and any potential adverse effects it may have in vivo. These results highlighted the importance of plant extract when taken with antibiotic medications. The Minimum Inhibitory Concentration (MIC) of Cinammon plant extracts was determined to be effective against Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii, respectively. The outcomes of the MIC are detailed in Table (3) below. The minimum inhibitory concentration (MIC) values for antibacterial activity demonstrated by Cinammon plant extracts against Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa ranged from 2.25 to 50 mg/ml. The degrees of antimicrobial activity displayed by the extracts examined varied according to the bacterial species that were put to the test. This would imply that only a very little amount of the extracts would be needed to stop the growth of the bacteria. According to the findings, there is a possibility that Cinnamon extracts could be utilized in the treatment of bacterial illnesses. Despite the need for clinical tests to determine the real viability of the treatment and whether or not it could have potentially fatal effects in vivo, the findings of this investigation were encouraging. These findings brought to light the significance of using plant extracts in conjunction with antibiotic medications for the purpose of bacterial control. In the current investigation, the plant extracts exhibited various synergistic abilities, each of which inhibited the growth of microbes to a varying degree. However, the zones of inhibition that occurred from the combination of antibiotics and plant extracts were higher than those that resulted from antibiotics alone. This was the case for all the microorganisms that were studied.
Conclusion
Based on the current study’s antibacterial assay, it was discovered that E. coli is more vulnerable to the Cinnamon plant extracts used than the other investigated microbes. According to the findings, plant extracts of Cinnamon have a lot of promise as antibacterial agents against microorganisms and can be applied to the management of infectious disorders brought on by resistant microbes. It is anticipated that future research would isolate the secondary metabolites from the extracts under consideration to specifically assess specific antibacterial activity and the hidden processes
Thus, the species fed more in the dry season to meet up with the increased metabolic demand linked with elevated temperature. The high gut repletion index (GRI) recorded by this species suggested that the species fed frequently and actively. This finding agrees with the reports of Ekpo et al. [18] in their separate studies. The wide food spectrum of P. elongatus is an indication of flexibility in trophic level which gives the species ecological advantage to feed effectively on different types of food based on availability of food items. The ability to exploit wide range of food supply by this fish invariably decreases the rate of competition for food between conspecifics and congenera [18]. P. elongatus depended mostly on autochthonous food items as other estuaries, creeks have been found to be very productive in terms of flora and fauna hence they have been described as breeding, nursery and feeding grounds for species [23]. Offem et al., [23] also reported that the ecological advantage of this is that it enables a fish species to alternate from one diet to another with regards to changes in the availability and abundance. This flexibility enhances the capability of the fish to make use of many different food items effectively and efficiently. The wide food spectrum of the species under study may be attributed to the high availability of this dietary items in the aquatic ecosystem regardless of the seasons so that the fish may have had unlimited access and consumed them according to their dietary needs [14,24].
References
- WHO (2013) World Health Report 2013: Research for Universal Health Coverage pp: 168.
- Edgar R, Domrachev M, Lash AE (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30(1): 207-210.
- Lai PK, Roy J (2004) Antimicrobial and chemo preventive properties of herbs and spices. Curr Med Chem 11(11): 1451-1460.
- Sulieman AE, Sherif MS, Ahmed A Alghamdi, Vajid NV, Mohanad A, et al. (2017) Evaluation of the antimicrobial and synergistic effect of selected medicinal plants in Hail area with antibiotic drugs. Bioscience Biotechnology Research Commun 10(1): 44-50.
- Sulieman AE, Abdallah EM, Alanazi NA, Ed-Dra A, Jamal A, et al. (2023) Spices as Sustainable Food Preservatives: A Comprehensive Review of Their Antimicrobial Potential. Pharmaceuticals 16(10): 1451.
- Gernot K (2007) Cassia Cinnamomum cassia (L)
- Zaidi SF, Muhammad A, Jibran Sualeh Muhammad, Makoto K (2015) Diverse pharmacological properties of Cinnamomum cassia: A Review: Pak J Pharm Sci 28(4): 1433-1438.
- Anandhi P, Tharani M, Rajeshkumar S, Lakshmi T (2022) Antibacterial activity of cinnamon and clove oil against wound pathogens. J Popul Ther Clin Pharmacol 28(2): e41-e46.
- Karar MG, Kuhnert N (2017) Herbal Drugs from Sudan: Traditional Uses and Phytoconstituents Pharmacogn Rev 11(22): 83-103.
- Talib WH, Alsayed AR, Abuawad A, Daoud S, Mahmod AI (2021) Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 26: 2506.
- Zhu D, Zhang Y, Wang S (2021) Histone citrullination: A new target for tumors. Mol Cancer 20(1): 90.
- Nabavi SF, Di Lorenzo A, Izadi M, Sobarzo-Sánchez E, Daglia M, et al. (2015) Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 7(9): 7729-7748.
- Nkuo-Akenji T, Ndip Roland, McThomas A, Chi Fru, Ernest (2001) Anti-Salmonella activity of medicinal plants from Cameroon. Cent Afr J Med 47(6): 155-1558.
- Gatsing D, Tchakoute V, Ngamga D, Kuiate JR, Tamokou JDD, et al. (2009) In Vitro Antibacterial activity of Crinum Purpurascens herb leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran J Med Sci 34(2): 126-136.
- Ahmed EM, Mirghani AY (2000) Sudanese medicinal plants used in folk medicine, (Gezira State, Sudan). The African Conference on Medicinal Plants Research, held at Elfatih University, Faculty of Pharmacy, Tripoli, Libya.
- Ahmed EM, Nour BYM, Mohamed YG, Khalid HS (2010) Antiplasmodial activity of some medicinal plants used in Sudanese folk-medicine. Environ Health Insights 4(4): 1-6.
- Khalid H, Abdalla WE, Abdelgadir H, Opatz T, Efferth T (2012) Gems from traditional north-African medicine: medicinal and aromatic plants from Sudan. Natural Products and Bioprospecting 2(3): 92-103.
- Demetriou EA, Lampit A, Quintana DS, Naismith SL, Song YJC, et al. (2018) Autism Spectrum Disorders: A Meta-Analysis of Executive Function. Mol Psychiatry 23(5): 1198-1204.
- Abdalla W, Abdallah E (2016) Promising Sudanese Medicinal Plants with Antibacterial Activity -a Review Article. Biological Forum 8: 299-323.
- Chokshi A, Sifri Z, Cennimo D, Horng H (2019) Global Contributors to Antibiotic Resistance. J Glob Infect Dis11(1): 36-42.
- Chinemerem ND, Ugwu MC, Oliseloke AC, Al-Ouqaili MTS, Chinedu Ikem J, et al. (2022) Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J Clin Lab Anal 36(9): e24655.






























