Abstract
Keywords:World Health Organization; Adenocarcinoma; Neoplasm; Metaplasia
Abbreviations: World Health Organization (WHO); NOS: Not Otherwise Specified; FAP: Familial Adenomatous Polyposis; FAP: Familial Adenomatous Polyposis; CT: Computerized Tomography; MRI: Magnetic Resonance Imaging; CEA: Carcinoembryonic Antigen; AFP: Alpha Fetoprotein
Introduction
Adenocarcinoma gallbladder is a carcinoma engendered from epithelium layering the gallbladder. Additionally designated as carcinoma of gallbladder, adenocarcinoma of gallbladder or malignant epithelial neoplasm of gallbladder, cholelithiasis appears as a major factor contributing to disease emergence. Neoplasm demonstrates an aggressive biological course and delineates an overall 5-year survival of <10%.
Adenocarcinoma gall bladder frequently arises within sixth to seventh decade. A female preponderance is discerned with female to male proportion of 3:1. Generally, female subjects from countries as Chile, Indian subcontinent, Pakistan or Ecuador are implicated. Neoplasm depicts an overall 5-year survival of <10% [1,2].
Adenocarcinoma gall bladder pre-eminently emerges within the fundus (~60%), body (~30%) or neck (~10%) of gallbladder. Advanced neoplasms appear to infiltrate hepatic parenchyma or extrahepatic biliary tree [1,2]. Adenocarcinoma gall bladder is posited to arise due to cholecystitis with cholelithiasis of extensive duration, conditions which induce intestinal or pseudo-pyloric subtype of mucosal metaplasia. Intestinal subtype of mucosal metaplasia induces epithelial dysplasia and carcinoma in situ. Disease progression from dysplasia to advanced adenocarcinoma may occur in up to 15 years [1,2].
Adenocarcinoma gallbladders appear concordant with conditions as chronic cholecystitis or hyalinising cholecystitis, cholelithiasis, porcelain gallbladder, obesity, bacterial infections, especially Salmonella or infestation with Opisthorchis viverrini, primary sclerosing cholangitis or pancreatico-biliary maljunction, particularly supra-Oddi adherence of common bile duct with main pancreatic duct. Occasionally, neoplasm may arise within subjects of Lynch syndrome or familial adenomatous polyposis (FAP) [1,2]. Commonly, tumour cells depict genetic alterations within TP53, CDKN2A, ARID1A, PIK3CA or CTNNB1 genes. Besides, genomic amplifications within ERBB2 or HER2 may ensue. Microsatellite instability (MSI) is observed within 10% instances.
Lesions confined to pancreatobiliary maljunction display chromosomal mutations within KRAS gene [2,3]. Clinically, neoplasm is preponderantly asymptomatic. Additionally, nonspecific clinical symptoms such as pain confined to right upper quadrant, loss of weight or pyrexia may concur. Overt clinical symptoms are associated with advanced disease [2,3]. Grossly, neoplasm may be discovered incidentally upon histological evaluation of cholecystectomy specimens. Macroscopically, tumefaction may be subtle or display foci of granular mucosa, irregular mucosa, minimal mucosal elevation, polypoid mucosal lesions or focal mucosal thickening within funds or body. Besides, thickened, indurated wall of gallbladder may be enunciated.Intracholecystic papillary neoplasm may exemplify exophytic or polypoid, friable mucosal lesions. Cut surface is firm, gritty, tan, grey/white or yellowish grey [3,4].
As per the contemporary World Health Organization (WHO) classification, adenocarcinoma gallbladder displays distinct histological variants categorized as ~biliary type adenocarcinoma as a predominant variant encountered in ~75% lesions and is constituted of adenocarcinoma not otherwise specified (NOS), papillary and micro-papillary variants. Akin to pancreatic ductal adenocarcinoma, neoplasm is comprised of tubules layered by cuboidal to columnar epithelial cells. Tubules appear enmeshed within a desmoplastic stroma. Of varied morphology ranging from bland countenance to poorly differentiated neoplasms, moderately differentiated tumours of adenocarcinoma gall bladder are commonly encountered [3,4].
Intestinal type adenocarcinoma is constituted of tubules layered by columnar epithelial cells pervaded with elongated, hyperchromatic nuclei. Tumours recapitulate colonic adenocarcinomas mucinous carcinoma is comprised of neoplasms displaying > 50% extracellular mucin clear cell carcinoma is constituted of sheets of clear cells demonstrating an alveolar pattern with tumour zones traversed by vascular articulations. Neoplasm resembles distant metastasis from clear cell renal cell carcinoma signet ring cell carcinoma preponderantly or singularly constituted of signet ring cells [3,4].
Hepatoid carcinoma wherein > 50% tumour is constituted of epithelial cells permeated with abundant, eosinophilic cytoplasm, enlarged nuclei and prominent nucleoli. Tumour depicts a preponderant trabecular pattern Sarcomatoid carcinoma or carcinosarcoma, which is predominantly composed of spindle shaped cells. Foci of heterologous differentiation as skeletal muscle, bone or cartilage may or may not be discerned [3,4]. Neoplasms with differentiation as well differentiated tumours composed of well-formed glandular articulations layered by columnar epithelial cells displaying minimal cytological anomalies.
Neoplastic glands appear confined within perimuscular connective tissue. Focal nuclear anomalies and mitotic figures may be discerned. Stromal desmoplasia is minimal moderately differentiated neoplasms comprised of irregular or angulated glandular configurations coated by polygonal tumour cells pervaded with enlarged nuclei with vesicular chromatin and prominent nucleoli. Mitotic figures are numerous. Stromal desmoplasia is significant. poorly differentiated neoplasms comprised of incomplete or poorly formed tubules, glandular structures, disseminated single cells or sheets of pleomorphic tumour cells pervaded with bizarre nuclei [3,4] (Figures 1 & 2) (Table 1).
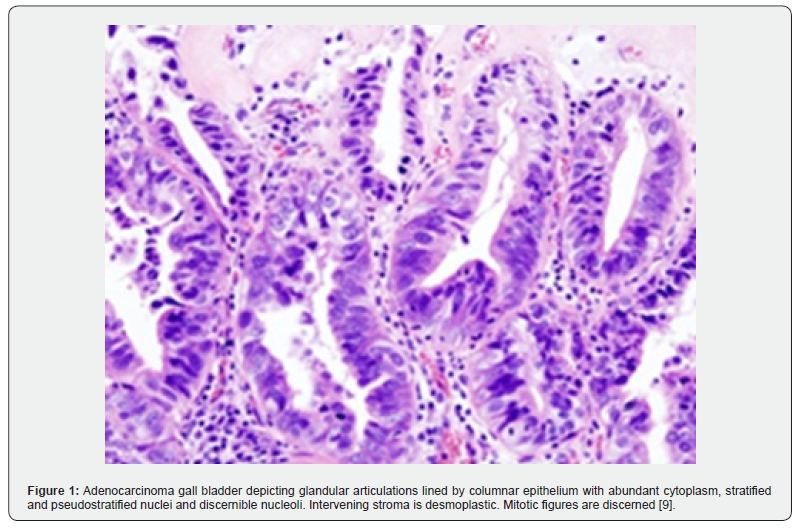
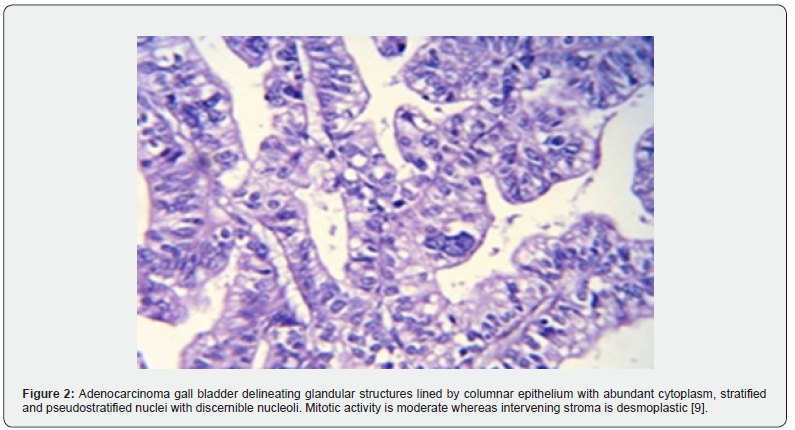
Adenocarcinoma gallbladder displays intense, diffuses immune reactivity to cytokeratin CK7, CK20, p53, carcinoembryonic antigen (CEA), CA19-9, IMP3, Mapsin, S100 protein, MUC1, HER2 or CDX2. Hepatoid variant appears immune reactive to Hep part1 and alpha fetoprotein (AFP). Tumour cells appear immune non-reactive to p16, PAX8, CD10 and CAIX [5,6]. Adenocarcinoma gallbladder requires segregation from neoplasms as pancreatic ductal adenocarcinoma, intrahepatic or extrahepatic cholangiocarcinoma or colonic adenocarcinoma [5,6]. Nearly 50% of lesions are discovered upon evaluation of cholecystectomy specimens wherein cogent macroscopic anomalies appear absent.
Radiographic features as a thickened gallbladder wall or polypoid lesions confined to the gallbladder necessitate prompt surgical intervention [5,6]. Advanced adenocarcinoma gallbladder is associated with elevated serum alkaline phosphatase. Sensitivity to carcinoembryonic antigen (CEA) is encountered in ~50% lesions [5,6]. Radiographic features may be subtle in preliminary disease or distinctive within delayed disease. Features such as thickening of gallbladder walls, mucosal elevation or polypoid lesion confined to gallbladder wall or lumen may be observed. Tumour may emerge as a mass occupying lesion or may replace gallbladder lumen [7,8].
Advanced neoplasms may configure a gallbladder mass infiltrating hepatic parenchyma. Ultrasonography emerges as a primary imaging modality for screening possible adenocarcinoma gall bladder. Computerized tomography (CT) may be beneficially adopted to assess anomalous features discerned upon ultrasonography. Magnetic resonance imaging (MRI) may be advantageously employed to obtain detailed information or evaluate specific tumour staging parameters [7,8]. Adenocarcinoma gall bladder may be suitably managed with surgical manoeuvers as cholecystectomy. Aforesaid surgical eradication along with or absence of tumour within cystic duct margin is appropriate in alleviating stage T1a neoplasms.
Additionally, procedures as hilar or portal lymphadenectomy along with resection of hepatic bed and common bile duct may be employed to exterminate tumour confined to surgical margins. Aforesaid manoeuvers appear suitable for treating tumours which extend into adjoining skeletal muscle or beyond and tumours within stage T1b toT3. Neoplasms associated with distant metastasis may be subjected to chemotherapy or radiation therapy. Irrespective of tumour magnitude or degree of differentiation, non-invasive papillary carcinoma appears devoid of distant metastasis. Invasive papillary carcinoma gallbladder is associated with superior prognostic outcomes [7,8].
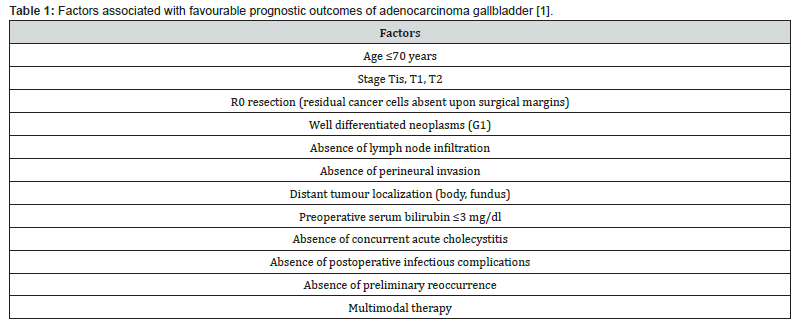
Prognostic outcomes of adenocarcinoma gallbladder not otherwise specified (NOS) are contingent to tumour stage wherein ~neoplasms with superficial invasion confined to lamina propria (stageT1a) delineate favourable prognostic outcomes and may be appropriately alleviated by cholecystectomy. ~advanced neoplasms associated with infiltration of skeletal muscle or beyond and tumours within stage T1b to T4 expound significant proportionate tumour reoccurrence and inferior prognostic outcomes. Poorly differentiated neoplasms of advanced histological grade with vascular invasion expound adverse prognosis. Tumour occurrence within Rokitansky-Aschoff sinus and margin of cystic duct emerge as possible predictors of tumour progression [7,8].
References
- Pavlidis ET, Galanis IN, Pavlidis TE (2024) New trends in diagnosis and management of gallbladder carcinoma. World J Gastrointest Oncol 16(1): 13-29.
- De Reuver PR, van der Post RS (2023) Clinicopathological and Molecular Insights into Gallbladder Cancer. Cancers (Basel) 15(10): 2728.
- Tarek Kellil, Mohamed Ali Chaouch, Emna Aloui, Mohamed Amine Tormane, Sahbi Khaled Taieb, et al. (2021) Incidence and Preoperative Predictor Factors of Gallbladder Cancer Before Laparoscopic Cholecystectomy: A Systematic Review. J Gastrointest Cancer 52(1): 68-72.
- Andrea Laurenzi, Giovanni Brandi, Federica Greco, Enrico Prosperi, Andrea Palloni, et al. (2023) Can repeated surgical resection offer a chance of cure for recurrent cholangiocarcinoma? Langenbecks Arch Surg 408(1): 102.
- Yanzhao Zhou, Kun Yuan, Yi Yang 3, Zemin Ji, Dezheng Zhou, Jingzhong Ouyang, et al. (2023) Gallbladder cancer: current and future treatment options. Front Pharmacol 14: 1183619.
- Reem K Shahin, Mohamed A Elkady, Ahmed I Abulsoud, Nourhan M Abdelmaksoud, Sherif S Abdel Mageed, et al. (2023) miRNAs orchestration of gallbladder cancer - Particular emphasis on diagnosis, progression and drug resistance. Pathol Res Pract 248: 154684.
- Gianluca Cassese, Ho-Seong Han, Yoo-Seok Yoon, Jun Suh Lee, Jai Young Cho, et al. (2022) Preoperative Assessment and Perioperative Management of Resectable Gallbladder Cancer in the Era of Precision Medicine and Novel Technologies: State of the Art and Future Perspectives. Diagnostics (Basel) 12(7): 1630.
- Kari Hemminki, Asta Försti, Otto Hemminki, Vaclav Liska, Akseli Hemminki (2022) Long-term incidence and survival trends in cancer of the gallbladder and extrahepatic bile ducts in Denmark, Finland, Norway and Sweden with etiological implications related to Thorotrast. Int J Cancer 151: 200-208.
- Image 1 and 2 Courtesy: Wikimedia commons.






























