Abstract
Primary thyroid lymphoma is an uncommon malignancy, accounting for 1-2% of extra-nodal lymphomas and 1-5% of thyroid malignancies. In which Diffuse large B-cell lymphoma is the most common type of primary thyroid lymphoma. It usually manifests as a rapidly enlarging neck mass with cervical lymphadenopathy. Primary treatment of most thyroid malignancies is surgery in most cases but in primary thyroid lymphoma treatment modality is totally different that is mainly via chemotherapy with or without chemotherapy, so early diagnosis is mandatory to start treatment on time. We present the case of a 55 years old female who presented with thyroid swelling which on histopathology and subsequent immunohistochemistry confirmed as high-grade B-cell lymphoma of the thyroid. She has received 6 cycles of chemo immunotherapy with R-CHOP with interim PET scan after 4 cycles showed DS:1; followed by ISRT, currently patient is on follow up and remained disease free [1].
Introduction
Primary thyroid lymphoma is relatively rare i.e. accounting for less than 5% of all thyroid malignancies. Among these cases, High-Grade B-cell lymphoma (HGBCL) represents a particularly aggressive subtype. HGBCL of the thyroid gland is characterized by its rapid clinical progression, poor prognosis, and challenging management. Given its rarity and unique clinical characteristics, understanding this entity is paramount to providing effective treatment strategies for patients. In recent years, there has been an increasing focus on studying the pathogenesis, molecular characteristics, and optimal treatment options for HGBCL of the thyroid gland. The aim of this case report is to contribute to existing knowledge by presenting a recent clinical example of HGBCL of the thyroid. By analyzing the available scientific evidence, we aim to enhance our understanding of this rare entity and provide insights into its presentation, diagnosis, management, and potential therapeutic options.
Case Presentation
55-year-old-female, presented in oncology OPD on 7 October 2023 with a history of neck swelling for 2.5 months. She has no history of weight loss, fever, and night sweats. On examination showed a mass over the thyroid extending more towards the right side, the mass moved with deglutition.
Previous workup Ultrasound Thyroid 3/10/23
Enlarged right lobe of the thyroid gland, which is measuring 58 x 43 x 40 mm. There is a large solid appearing heterogeneous predominantly hypoechoic nodule within the right lobe of the thyroid with ill-defined margins. It shows no internal calcifications or vascularity on colour Doppler. It is abutting and displacing the adjacent structures. Displacement of the trachea towards the left side and displacement of the carotid sheath towards the right side. Distended internal jugular vein in the region of enlarged thyroid lobe with internal heterogeneous material filling its lumen likely representing chronic thrombosis. Multiple enlarged hypoechoic lymph nodes with loss of fatty hilum at level IV, largest measures 10mm in short axis. Left thyroid gland is also mildly enlarged measuring 45 x 20 x 17 mm. It shows a heterogeneous mixed echogenicity nodule with irregular margins and spongiform appearance measuring 10-x 5 mm. Enlarged thyroid isthmus infiltrated by the right thyroid lobe mass measuring 11 mm in thickness. Small anechoic nodule measuring 7 X 4 mm. IMPRESSION: TI-RADS 5 lesion in right thyroid lobe with ipsilateral level IV lymphadenopathy and chronic internal jugular vein thrombosis. TI-RAD 2 lesion in left lobe of thyroid.
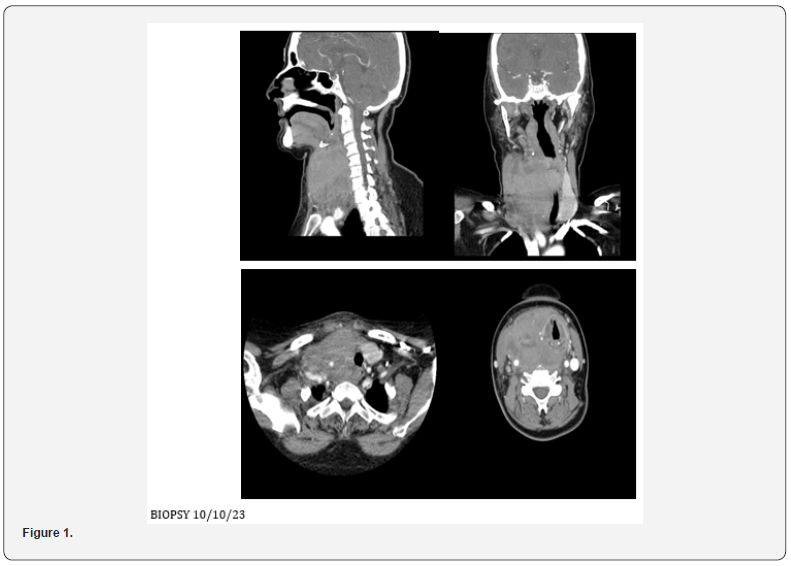
Ct Neck Contrast 4/10/23
• Large solid enhancing lesion is identified arising from
the right lobe of thyroid gland, which is completely replaced by
the lesion and is extending into the isthmus. Few necrotic areas
identified within the lesion along with few specks of calcification.
The lesion measures approximately 57 x 72 x 95 mm.
• Minimal retrosternal extension identified.
Anteriorly the lesions infiltrating the strap muscles and right
sternocleidomastoid muscle. Posteriorly it involves prevertebral
fascia. Laterally it is encasing the trachea and displacing it
towards the left side however, no significant tracheal narrowing
is noted. Bilateral thyroid cartilage is also involved in the lesion. It
is also compressing and displacing the esophagus towards the left
side. The lesion is also encasing the right common carotid artery.
Superiorly it is reaching up to the level of hyoid bone. The right
piriform fossa and vallecula are effaced due to mass effect. The
posterior wall of the hypopharynx appears to be infiltrated by
the lesion. The right brachiocephalic vein is also encased by the
lesion. The internal jugular vein is significantly attenuated.
• Few enlarged cervical lymph nodes are noted, largest
one is noted in right level IIb, measuring approximately 13 mm
in short axis. Left lobe of thyroid gland appears normal and is
significantly displaced towards the left side. Significant fat atrophy
of bilateral submandibular glands is noted. Mild fatty replacement
is also noted in bilateral parotid glands. Tongue and floor of mouth
appear normal. Nasopharynx appears normal. Epiglottis and
fossae of Rosen Mueller appear normal. Paranasal sinuses and
mastoid air cells appear clear (Figure 1).
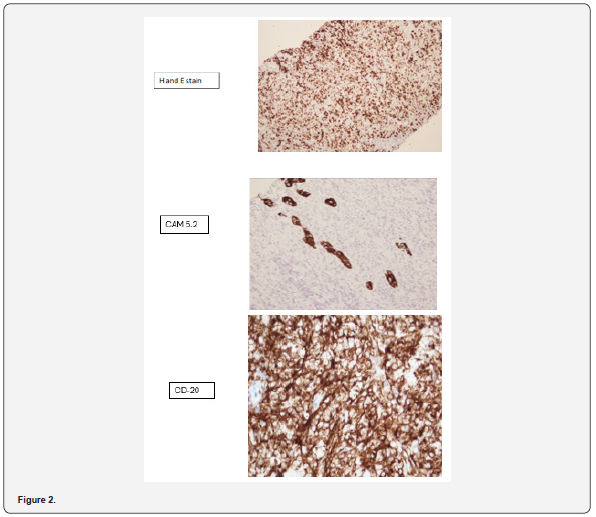
BIOPSY 10/10/23 Gross Description
• The specimen is received in a single formalin container coded as “Right thyroid lobe mass core biopsy”. It consists of two tan-white linear cores measuring 1.9 x 0.2 cm in aggregate. Entirely submitted in a single cassette.
Microscopic Description
• Sections examined reveal linear cores of tissue involved
by diffuse infiltrate comprising of partly crushed cells which
appear intermediate to large present in sheets interspersed
by fibrotic stroma. In relatively preserved areas nuclei show
vesicular chromatin. Occasional nucleoli are also seen. There are
skeletal muscle bundles entrapped. Immunohistochemical stains
performed show the following reactivity pattern:
• CD20 Positive
• BCL2 Positive
• CD10 negative
• BCL6 negative
• Mum-1 Positive
• C-Myc Positive
• Mib-1 approximately 70%
• Cytokeratin CAM 5.2 Highlights occasional native thyroid
follicles.
• PAX8 Positive in occasional native thyroid
• CD5 negative (Figure 2)
Diagnosis
• Right thyroid lobe mass, core biopsy: High-grade B-cell
Lymphoma. Differentials include Diffuse Large B-cell Lymphoma,
Non-Germinal center type
• MYC and BCL2 Re-arrangement by FISH not detected.
Pet Scan 18/10/23
Head and Neck: There is evidence of normal FDG distribution
over the brain cortex with no evidence of mass effect but some
misregistration artifact. Para-nasal sinuses are clear. A large
hypermetabolic confluent mass involving right lobe of thyroid and
pushing the tracheal towards left, crossing midline and involving
left lobe. The mass extends inferiorly to suprasternal notch and
superiorly to right pyriform sinus. Mass is abutting the right
lamina of thyroid cartilage. Mass is measuring 60 x 80 x 104 mm
(TR x APx CC) and SUV smax 2.4.0 on right (57 x 72 x 95 mm in last
CT) and 26 x23mm and SUV max 14.9 on left lobe. Hypermetabolic
nodes are seen in right cervical lev
el II region (largest measuring
13 x 10 mm and SUV max 8.4).
Chest: Both breasts show no evidence of hypermetabolic
focus. No evidence of hypermetabolic node in axilla, hilar or
mediastinal regions. A tiny calcified granuloma is seen lower lobe
of right lung. Rest of the structure in chest shows physiological
tracer distribution with normal morphology. There is no evidence
of pulmonary nodules on FDG or CT images.
Abdomen And Pelvis: Uniform tracer distribution is seen
over liver (172 mm CC) and sp. (120 mm CC). A tiny radiopaque
gallstone is seen. No evidence of hypermetabolic nodes in
abdomen or pelvic region is noted. Both adrenals and pancreas
are within normal limits. Physiological tracer distribution is seen
in bowel and urinary tract.
Skeletal: Mild scoliotic deformity is seen involving lumbar
spine with age-related degenerative arthritis changes. No
evidence of FDG avid marrow or skeletal metastasis.
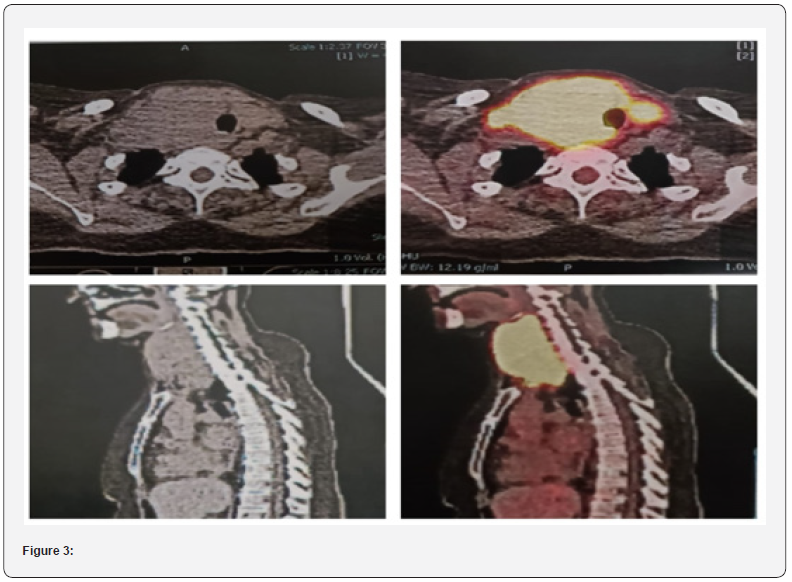
Lugano Classification: Stage I-E
• UCE: 28/0.8/146/4/104/23
• Hemoglobin 14.5G/DL/
• TLC 8990 CMM (neutrophils 85.6%, Lymphocytes 9.7%)
• Platelets 204CMM
• LFT: T.BILI 0.44 MG/DL ALT 13 U/L, AST 16 U/L, ALP
106 U/L, GGT 39 U/L
• LDH 322U/L (240-480)
• URIC ACID 2.8
• PO4 3 MG/DL
• CAL 9 MG/DL
• APTT 25.9
• INR 1.1
• ALBUMIN 4.2
• VIRAL SEROLOGY NEGATIVE, HEP B CORE AB N/R
• ECHO: EF 65%, NORMAL LV FUNCTION, GRADE I LVDD
Impression Stage IE PRIMARY THYROID LYMPHOMA
Diffuse large B-cell lymphoma (non-germinal center subtype)
• IPI 0 / bulky disease
• Planned for R-CHOP X 6 cycles followed by XRT
• MYC and BCL2 Rearrangement by FISH not detected.
The patient started with chemo-immunotherapy with an R-CHOP regimen and received 6 cycles till now with interim PET scan sowed complete metabolic response, followed by involved site radiation with 36Gy. Currently patients are disease free and remained on follow up with history and physical examination and laboratory workup includes Complete blood profile, LDH level, TSH level and calcium level monitoring. Height- 154 CM, WEIGHT 62KG, BSA 1.62
Discussion
In terms of geographic distribution, ethnic variation, anatomical localization, etiology, and morphological diversities, primary extranodal lymphoma (pENL) is a heterogeneous illness [1]. In areas with high overall lymphoma incidence, primary extranodal NHL frequency is high. An incidence of 44% (106/241 cases over a three-year period) was observed in research conducted in North India by Singh et al. [2]. In a similar vein, Padhi et al.’s South Indian study [3] recorded an incidence of 22%. Research from China [6], Korea [5], Pakistan [4], and Korea [5] have also documented instances that range from 45% to 62%. Primary thyroid lymphoma (PTL) is very rare, accounting for 1% to 8% of thyroid malignancies and 1% to 7% of all extranodal lymphomas [7,8]. The most common type of PTL is diffuse large B-cell lymphoma (DLBCL), followed by mucosa-associated lymphoid tissue (MALT) lymphoma. Hashimoto thyroiditis (HT) is the only well-known risk factor for developing PTL, However, only 0.6% of those with thyroiditis develop lymphoma [9,10]. Primary thyroid lymphoma is a rare disease that continues to produce diagnostic and therapeutic dilemmas. PTL occurs 4 times more often in women than in men. It usually occurs in the 7thdecade age group, with the median age being 67 years, while PTL is common in the middle-aged group with an average age of 40 years. Usually, patients with PTL have a history of painless thyroid swelling within 1-3 months that is associated with dyspnea, stridor, dysphagia, hoarseness, cough, and superior vena cava obstruction. It can be associated with B symptoms such as fever, night sweats, and weight loss in 10-20% of patients [10]. The prognosis is relatively good for PTLs, as most are indolent. However, relapses occur in 6% to 26% of patients, always leading to poor outcomes. Since prognosis depends considerably on stages and subtypes, early diagnosis is important..
Most commonly, ultrasound (US) of PTL shows a hypoechoic mass, with echogenicity less than that of the adjacent neck musculature, combined with hypervascularity and a characteristically undifferentiated outline. Computed tomography (CT) and magnetic resonance imaging (MRI) show a homogenous mass with a lack of calcification, cystic degeneration, and necrosis. Typical imaging demonstrates homogenous, mild enhancement, and mild T2 hyperintensity compared with the surrounding thyroid gland. Currently, positron emission tomography (PET)-CT plays an important role in the detection and staging of PTL. PETCT images show increased uptake of fluorodeoxyglucose (FDG) in the thyroid lobe, with/without lymph node involvement, and help to stage, restage, or evaluate the response to treatment. Bone marrow biopsy should also be performed to rule out marrow involvement. Noninvasive methods are recommended in the cases of a suspicious mass. FNA under US guidance can confirm the diagnosis for 70-80% of patients, but for low-grade lymphomas, especially when the distinction between MALT and HT is quite difficult, the distinguishing features may be the abundance of lymphoid tissue and a high proportion of intermediate centrocytelike cells in low-grade PTL compared to HT. With the help of flow cytometry or immunohistochemical staining, the accuracy of FNA is increasing. Core or open surgical biopsy is still essential when the diagnosis resulting from FNA is obscure or raises suspicion of rare subtypes. Moreover, researchers still recommend that all patients undergo open excisional biopsy to make a definitive assessment of the exact subtype or stage of PTL. Establishing the diagnosis is important as lymphoma is managed by chemotherapy, while carcinoma is managed by surgical resection if the tumor can be resected [11-13].
Traditionally, surgery and radiation therapy (RT) have been considered standard treatments for PTL. However, with high relapse and low survival rates, and the realization that thyroid lymphomas are responsive to chemotherapy and radiation, surgery now plays a limited role. According to studies from the Mayo Clinic, high cure rates and disease-free survival are achieved with thyroidectomy and adjuvant RT in thyroid lymphoma. Currently, the role of surgery is to obtain more tissues for histological diagnosis. Cervical-mediastinal RT should be the initial treatment of choice for patients with a good prognosis and when the disease is limited to the thyroid. In a case study of 31 patients with primary thyroid MALT lymphoma, a 5-year survival rate of 90% was observed after RT alone. Chemotherapy should be performed together with radiotherapy. for high-grade thyroid lymphomas that show extracapsular extension. The R-CHOP regimen that contains rituximab has been demonstrated to be the best combination therapy for disease-free survival in high-grade thyroid lymphoma mostly DLBCL [14-16]. R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard first-line treatment for most DLBCL. Rituximab is the first anti-CD20 monoclonal antibody approved by the FDA for lymphoma treatment which promotes tumor killing through direct induction of apoptosis, antibody binding-induced complement-dependent cytotoxicity (CDC), and antibody-dependent cytotoxicity (ADCC) mediated immune response. patients with DLBCL may be divided into two types by the Hans classification, such as GCB type and non-GCB type. Studies have shown that the majority of patients with primary extra-nodal DLBCL are of the non-GCB type. Furthermore, the GCB type is correlated with a better prognosis than the non-GCB type. Studies have shown that most of the primary thyroid dlbcls were of nongcb type which is in line with our current case [16,17].
The overall prognosis of extranodal DLBCLs appears to be inferior to that of nodal DLBCL, while the prognosis of extranodal DLBCLs arising in different sites varies greatly. Until recently, baseline prognostic evaluation has centered on the International Prognostic Index (IPI), which is assessed on five dimensions including age, general condition, clinical stage, lactate dehydrogenase (LDH), and extranodal involvement. In some studies, for primary extranodal DLBCLs, some sites related to high-risk for poor outcomes were listed, and the results are not entirely consistent. For example, a US registry-based analysis classified that primary extranodal DLBCLs of the gastrointestinal tract, lung, and liver/pancreas showed poor prognosis, while primary DLBCLs of the head and neck predicted good survival. Another series of 1,085 patients from China showed that primary extranodal DLBCLs arising from stomach, breast, sinus, lung, and salivary gland sources had a good prognosis, while those from CNS, testis, oral, and kidney sources had an inferior survival.7 The overall risk of CNS recurrence in DLBCL is lower than 5%, while involvement of certain extra-nodal sites is a risk factor for CNS recurrence, such as the testes, kidneys, and adrenal glands. Compared with other extranodal DLBCLs, the Ann Arbor clinical stage of PTL is usually limited to the early stage, with most of them in stage I or II. Therefore, PTL including thyroid DLBCL has a good prognosis. Modern chemotherapy and radiotherapy combined with rituximab have shown good results in clinical treatment of PTL, and surgery is no longer the preferred treatment for PTL [16,17]. Our case highlights that a rapidly growing mass in thyroid gland must not only raise the suspicion of primary thyroid malignancy but a rarity like primary thyroid lymphoma, must also be kept as a important differential diagnosis. Prompt diagnosis and initiating earliest possible treatment may improve outcomes. one must not proceed with thyroidectomy upfront of a suspicious mass in thyroid as in ptl surgery is mostly not required. Due to the low incidence rate of primary extranodal lymphoma in certain sites specifically in thyroid, the relevant research is limited, which hinders researchers from obtaining more information on their biology. More basic studies and prospective clinical trials are needed to clarify the molecular mechanisms and clinical characteristics of extranodal lymphoma and guide the selection of clinical treatment options.
Conclusion
This case report on High-Grade B-cell lymphoma (HGBCL) of the thyroid gland highlights the challenges associated with the diagnosis, management, and prognosis of this rare entity. Through the analysis of the presented case and referencing recent scientific literature, a comprehensive overview of HGBCL has been provided. While HGBCL remains an aggressive malignancy with a poor prognosis, advances in molecular profiling and immunotherapeutic approaches offer hope for improved outcomes. Further research is needed to elucidate the underlying genetic and molecular alterations contributing to HGBCL development, as well as to explore targeted therapies that may provide more effective treatment options. By sharing our findings and insights on HGBCL, we hope to contribute to the collective understanding of this rare malignancy and guide future research endeavors. Through continued collaboration and research, it is our goal to pave the way for improved diagnostic accuracy, therapeutic strategies, and ultimately, better outcomes for patients with HGBCL of the thyroid gland.
References
- Anderson JR, Armitage JO, Weisenburger DD (1998) Epidemiology of the non-Hodgkin's lymphomas: Distributions of the major subtypes differ by geographic location. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol 9(7): 717-720.
- Singh D, Kumar L, Goyal H, Raina V, Bijlani L, Wadhwa J (2003) Primary extranodal non-Hodgkin's lymphoma in northern India. Proc Am Soc Clin Oncol 22: 2457.
- Padhi S, Paul TR, Challa S, Prayaga AK, Rajappa S, et al. (2012) Primary extra nodal non-Hodgkin lymphoma: A 5-year retrospective analysis. Asian Pac J Cancer Prev 13(10): 4889-4895.
- Nagi AH, Al Minawy L, Naseem N, Henna SN, Naveed IA (2010) A study of the morphological patterns of extranodal non-Hodgkin lymphoma in Pakistani and Saudi populations. Biomedica 26: 118-123.
- Yoon SO, Suh C, Lee DH, Chi HS, Park CJ, Jang SS, et al. (2010) Distribution of lymphoid neoplasms in the Republic of Korea: Analysis of 5318 cases according to the World Health Organization classification. Am J Hematol 85(10): 760-764.
- Yang QP, Zhang WY, Yu JB, Zhao S, Xu H, et al. (2011) Subtype distribution of lymphomas in Southwest China: Analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol 6: 77.
- Widder S, Pasieka JL (2004) Primary thyroid lymphomas. Curr Treat Options Oncol 5(4): 307-313.
- Pedersen RK, Pedersen NT (1996) Primary non-Hodgkin's lymphoma of the thyroid gland: a population-based study. Histopathology 28(1): 25-32.
- Widder S, Pasieka JL (2004) Primary thyroid lymphomas. Curr. Treat. Options in Oncol 5(4): 307-313.
- George H Sakorafas, Panayiottis Kokkoris, David R Farley (2010) Primary thyroid lympoma: Diagnostic and therapeutic dilemmas 19(4): e124-e129.
- Diaconescu MR, Costea I, Glod M, et al. (2016) An Unwonted Clinicopathological Subtype of Thyroid Primary Lymphoma. Chirurgia (Bucur) 111(5): 428-431.
- Aiken AH (2012) Imaging of thyroid cancer. Semin Ultrasound CT MR 33(2): 138-149.
- Sakorafas GH, Kokkoris P, Farley DR (2010) Primary thyroid lymphoma (correction of lympoma): diagnostic and therapeutic dilemmas. Surg Oncol 19(4): e124-129.
- Doria R, Jekel JF, Cooper DL (1994) Thyroid lymphoma. The case for combined modality therapy. Cancer 73(1): 200-206.
- KJ Harrington, VJ Michalaki, L Vini, CM Nutting, KN Syrigos, et al. (2005) Management of non-Hodgkin's lymphoma of the thyroid: the Royal Marsden Hospital experience. Br J Radiol 78(929): 405-410.
- CM Pyke, CS Grant, TM Habermann, PJ Kurtin, JA van Heerden, et al. (1992) non-Hodgkin’s lymphoma of the thyroid: is more than biopsy necessary? World J Surg 16: 604-609.
- Zhimin Bai, Lingyu Li, Tao Guan, Jiangtao Wang, Jin Zhao, et al. (2021) Clinical prognosis and bioinformatic analysis of primary thyroid lymphoma. Medicine (Baltimore) 100: e24598.
- George H Sakorafas, Panayiottis Kokkoris, David R Farley (2010) Primary thyroid lymphoma - diagnostic and therapeutic dilemmas SurgOncol 19(4): e124-129.
- Maria Bai, Angelos Skyrlas, Niki J Agnantis, Sevasti Kamina, Alexandra Papoudou-Bai, et al. (2005) B-cell differentiation, apoptosis and proliferation in diffuse large B-cell lymphomas. Anticancer Res 25(1A): 347-362.
- Sehn LH, Salles G (2021) Diffuse large B-cell lymphoma. N Engl J Med 384(9): 842-858.
- Alizadeh AA, Eisen MB, Davis RE, et al. (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403(6769): 503-511.
- Castillo JJ, Winer ES, Olszewski AJ (2014) Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: an analysis of the Surveillance, Epidemiology and End Results database. Am J Hematol 89(3): 310-314.
- Sabela Bobillo, Erel Joffe, Jessica A Lavery, David Sermer, Paola Ghione, et al. (2021) Clinical characteristics and outcomes of extranodal stage I diffuse large B-cell lymphoma in the rituximab era. Blood 137(1): 39-48.






























