Investigating Clinicopathologic Characteristics and Outcomes in Soft Tissue Sarcoma Patients: Insights from a Tertiary Care Setting
Shumyla Beg1, Aiman Majid2*, Naila Zahid3, Iram Qureshi4, Hira Khan Afridi5 and Navaira Ali6
1Assistant Professor Oncology department, JPMC, Pakistan
2Senior Registrar Oncology Department, LNH, Pakistan
3Head of Oncology Department, LNH, Pakistan
4Medical Officer Special Ward, LNH, Pakistan
5Assistant Professor Oncology Department, LNH, Pakistan
6Medical Officer Oncology Department, LNH, Pakistan
Submission: December 30, 2024; Published: January 16, 2025
*Corresponding Address:Aiman Majid, Department of Oncology, Liaquat National Hospital, Karachi, Pakistan
How to cite this article:Shumyla Beg, Aiman Majid, Naila Zahid, Iram Quershi, Hira Khan Afridi and Navaira Ali. Investigating Clinicopathologic Characteristics and Outcomes in Soft Tissue Sarcoma Patients: Insights from a Tertiary Care Setting. Canc Therapy & Oncol Int J. 2025; 28(2): 556232. DOI:10.19080/CTOIJ.2025.28.556232
Abstract
Background:Soft tissue sarcoma (STS) can develop virtually anywhere in the body, however, most tumors originate in extremity, trunk, retroperitoneum, or head and neck. More than 50 different histologic subtypes of STS have been identified. The most common are pleomorphic sarcoma, GIST, liposarcoma, leiomyosarcoma, synovial sarcoma, and malignant peripheral nerve sheath tumors. Rhabdomyosarcoma occurs most commonly in childhood.
Objective:To determine the clinicopathologic characteristics and their outcomes among soft tissue sarcoma patients at a tertiary care hospital in Karachi.
Material and Methods: This study was a retrospective observational study which was conducted among patients who visited Oncology Department, Liaquat National Hospital, Karachi, Pakistan, from 2010 to 2022. Clinical stage, presenting complaints and overall survival of 90 included patients were recorded. Survival and recurrence rates were assessed using telephone-based data collection approach. Fisher’s exact test and chi-square were used to examine associations between qualitative variables. Log rank Kaplan-Meier analysis was used for survival analysis. P-value <0.05 was considered significant.
Results: The included patients had an average age of 41.44±16.71 years. The majority of the patients were in the 51–60 age range. There were 45.6% female patients and 54.4% male patients. Grade-III with 57.8% was most observed grade. 74.4% had localized stage and 25.6% had metastatic stage. 31.3% received their first line, 10.9% received second line, and 4.7% received third line chemotherapy. Death and recurrence rates were 36.7% and 41.1%, respectively. Ewing sarcoma, GIST, and leiomyosarcoma were detected in 31.1%, 31.1%, and 13.3% respectively. Survival rate at 2 years was 50% for GIST, 33% for leiomyosarcoma, 28.6% for ewing sarcoma and 31% for another sarcoma.
Conclusion: The study highlighted the local demographics and outcomes of the rare diseases of STS. The outcomes are comparable to those of the developed world.
Keywords:Clinicopathologic Characteristics; Outcomes; Soft Tissue Sarcoma
Introduction
Rare connective tissue malignant tumors called sarcomas are characterized by their heterogeneity and the ability to differentiate into several cell types, including those of other connective tissues (lipocytes, fibrous supporting structures, muscle, etc.), visceral tissues, and bone [1]. Soft tissue sarcomas (STSs) are tumors arising from mesodermal portion of the embryo that develops into connective and skeletal tissues. They are uncommon tumors comprising less than 1% of all malignant tumors [2]. Although rare, these are life threatening and cause significant diagnostic and therapeutic challenges [2]. As much as 40% of all STS develop in the extremities, to be precise, lower extremities more often than upper extremeties (28% vs 12%). About 44% of all STS involving an extremity occur in the thigh [3]. Genetic and environmental variables (such as chemical carcinogens), irradiation, viral infections (especially HHV-8), and immunological weakness have all been linked to an increased risk of STS, although its exact cause remains unknown [4].
STS is a diverse collection of malignant tumors that includes >100 distinct histologic subtypes [5]. These tumors are most frequently observed in the retroperitoneum, extremities, trunk, and head and neck area [6]. The STS spectrum includes different neoplastic histotypes [7,8] classified basically by their (putative) cell lineage of differentiation as adipocytic, fibroblastic/ myofibroblastic, fibrohistiocytic, smooth muscle, pericytic, skeletal muscle, vascular or chondro-osseous neoplasms; nerve sheath tumors; tumors of uncertain differentiation; or undifferentiated/ unclassified sarcoma [9]. The most common histological subtypes are leiomyosarcomas (14%) and liposarcomas (10%); while some of STSs (16%) lack any consistent lineage of differentiation (socalled unclassified sarcomas) [10].
There is no single clinical examination to facilitate the accurate diagnosis of soft tissue sarcomas, which requires comprehensive consideration of several examinations. Clinical histories and findings from physical examination are sometimes useful in the diagnostic process of soft tissue tumors. Moreover, imaging modalities, especially magnetic resonance imaging (MRI), are powerful tools for the diagnosis of soft tissue tumor [11]. Histology is used to provide the definitive diagnosis of STS, ruling out the possibility of a benign growth and, if cancerous, determining the sarcoma’s histological grade as well as subtypes [12]. When a mass cannot be identified by the use of clinical history, physical examination, laboratory tests, or imaging, followed by biopsy may be required to make a definitive diagnosis. Biopsies are performed to gather diagnostic tissue while minimizing collateral damage, stopping the spread of cancer, and interfering with future treatment options. Some methods that have been developed to achieve these aims are open surgical biopsy, fine-needle aspiration (FNA) as well as core biopsy [13].
Sarcomas generally have a poor prognosis, with a five-year overall survival rate hovering around 36%-58% [14]. Overall survival is impacted by various prognostic factors that include histologic subtype, grade, and completeness of tumor resection [15]. Determination of the optimal treatment can be complex and ideally requires a team of dedicated specialists, including surgeons, medical oncologists, radiation oncologists, pathologists, and radiologists [16]. STSs are usually somewhat resistant to system therapies such as chemotherapies, which is why distant metastases often occur during the course of the disease, which ultimately have a decisive influence on survival. Therefore, appropriate radical local therapy is of utmost importance for the cure of STS [17]. One challenge in the curative treatment of STS is diffuse local microscopic spread and extension of the tumor to nerves and vessels. Therefore, radiotherapy plays an important role in the multimodal therapy of high-risk STS to achieve longterm local control [18]. Not much data has been published regarding the clinicopathologic features and outcomes of soft tissue sarcomas in Pakistan. Due to the paucity of data elucidating the outcomes of STS in developing nations such as Pakistan, there is a critical need to analyze these parameters as they pertain to STS. Managing STS in a developing country is a challenge. Limited financial resources do not allow patients to come early to tertiary care centers. The present study therefore aims to delineate these parameters includes the characteristics and its outcomes.
Material and Methods
This study was a retrospective observational study which was conducted among patients who visited Oncology Department, Liaquat National Hospital, Karachi, Pakistan, from 2010 to 2022. The study was conducted after obtaining approval from the ethical review committee of the institute. Ethical considerations were paramount throughout the study, and the research protocol was reviewed and approved by the relevant Institutional Review Board. Verbal consent was obtained from each participant before proceeding with the data collection process. The purpose of the study, as well as the voluntary nature of their participation, was clearly explained to ensure their understanding and agreement to take part. We have found total 90 patients with biopsy proven soft tissue sarcoma over a period of 13 years. The patients of both genders with age more than 18 years had histopathologically proven diagnosis of soft tissue sarcoma of any histology and continued treatment and visited for follow up too were included in the study. To maintain the focus on a single institutional study patients who had received treatment from multiple institutions were not included in the study.
Data collection was done from the institute’s electronic health records (EHR) system and Health Information Management System (HIMs). The dataset included information on patient demographics, Sarcoma subtype, stage at diagnosis, treatment received, and follow-up details. Clinical features including specific histology of the tumor at the time of diagnosis and Eastern Cooperative Oncology Group (ECOG) Performance Status (ECOG) of the patients before treatment were recorded. Clinical stage at presentation and the presenting presenting complaints of the patient were noted too. Personal details like names, age at diagnosis, gender and family history of any cancer in the family of patients was recorded. Overall survival minimum of one year would be calculated. The diagnosis of cancer was based on the histopathological analysis of the patient’s biopsy specimen from any recognized laboratory in the country. and follow up data was used to calculate recurrence and overall survival. To assess the overall survival and recurrence rates in soft tissue sarcoma patients who no longer required follow-up, a telephone-based data collection approach was employed. This method involved contacting individuals whose records indicated that they had been treated for sarcoma at this institution, specifically during the period spanning from 2010 to 2022. A trained research team conducted the telephone interviews using a structured questionnaire.
The questionnaire was designed to capture pertinent information related to the participants’ current disease status and living conditions. This allowed us to gather data on survival outcomes and the disease recurrence. During the telephone interviews, participants were asked about their current health status, including any signs or symptoms of disease progression or recurrence. Additionally, information regarding their overall wellbeing, quality of life, and any ongoing treatments or interventions was also collected. The data was analyzed using SPSS version 23. Qualitative variables such as gender, ethnicity and other relevant basic information related to cancer site, staging and age were expressed as frequency and percentage. Quantitative variables such as age were expressed as mean and standard deviation. Fisher’s exact test and chi-square were used to examine any associations between the qualitative variables. Log rank Kaplan- Meier analysis was used for survival analysis. P-values below 0.05 were regarded as statistically significant.
Results
The included patients had an average age of 41.44±16.71 years. The majority of the patients were in the 51-60 age range. There were 45.6% female patients and 54.4% male patients. Overall, ninety patients, 4.4% have a positive family history of cancer and ninety-eight percent have a good socioeconomic standing. Primary site discomfort (56.7%), swelling/mass (27.8%), and bleeding (11.1%) were the most frequent presenting complaints. The detailed results are presented in (Table 1). Total 35.6% patients had functional morbidity. Grade-I was observed in 28.9% patients, grade-II in 13.3%, and grade-III in 57.8% patients. Out of the 90 patients, 74.4% had a localized stage and 25.6% had a metastatic stage. The most frequent metastatic sites were liver 26.1% and lung 39.1%. For 76.7% patients, the intention of the treatment was curative, while for 23.3%, it was palliative. The detailed results are presented in (Table 2).
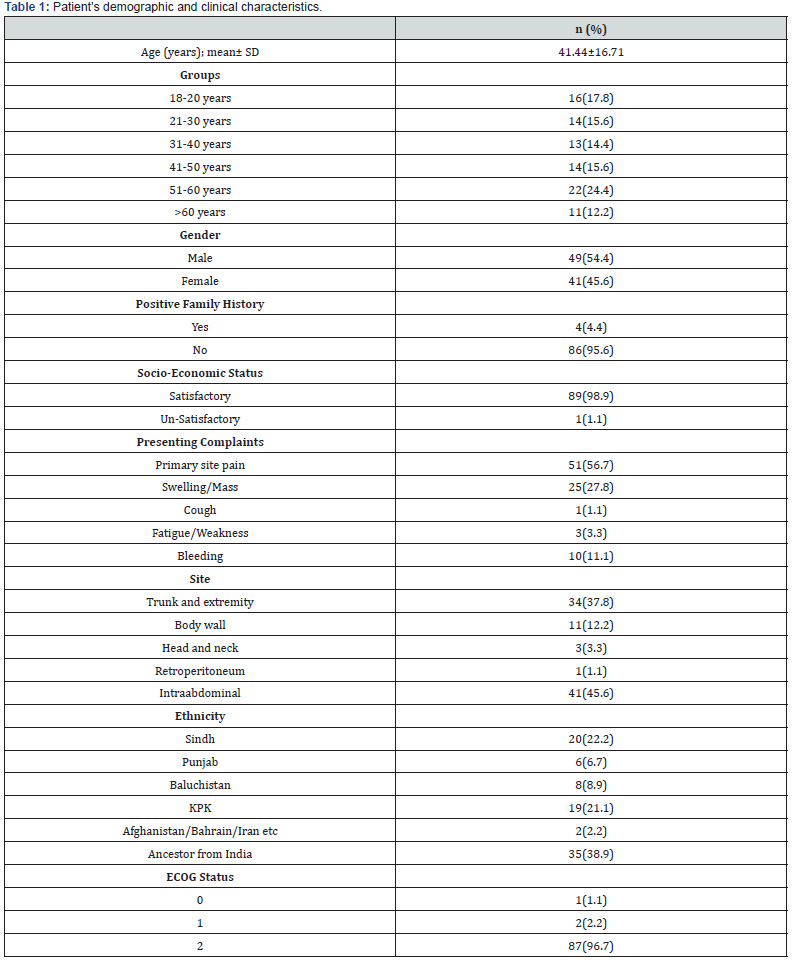
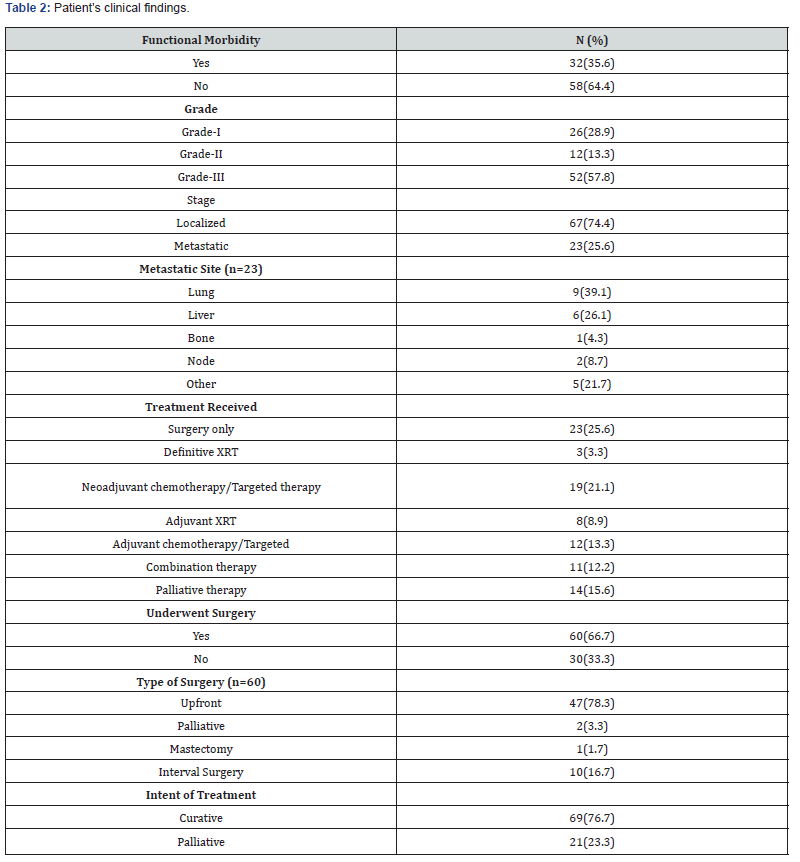
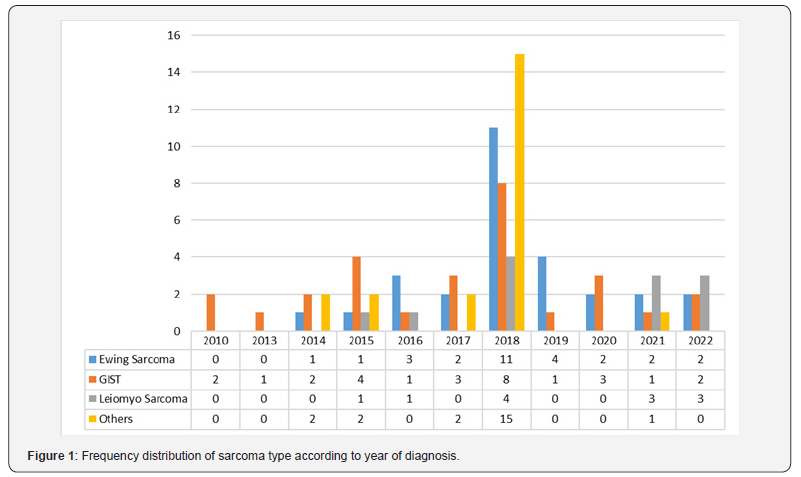
Total 64 patients received chemotherapy; of these, 31.3% received their first line, 10.9% received their second line, and 4.7% received their third line. In our study, the death rate was 36.7%, and the recurrence rate was 41.1%. Ewing sarcoma, GIST, and leiomyosarcoma were detected in 31.1%, 31.1%, and 13.3% of the patients in our study respectively. Table 3 displays the comprehensive results of chemotherapy and its outcomes. Figure 1 shows the frequency distribution of sarcoma types according to the year of diagnosis.
Table 4 represents association of sarcoma with patient’s demographic to observe the statistically significant factors. It was observed that age group (p=0.000), presenting complaints (p=0.001), site (p=0.000), functional morbidity (p=0.003) was found to be significantly associated with the type of sarcoma.
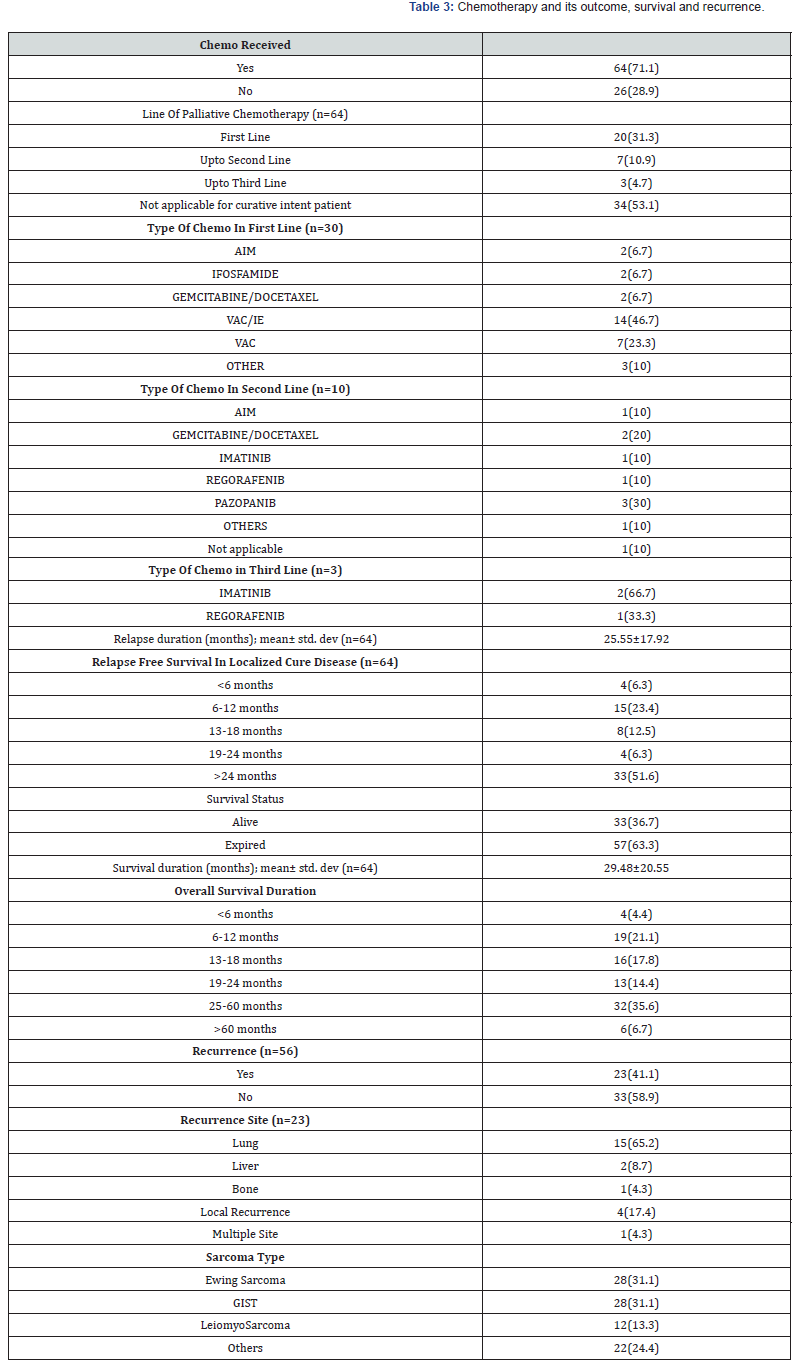
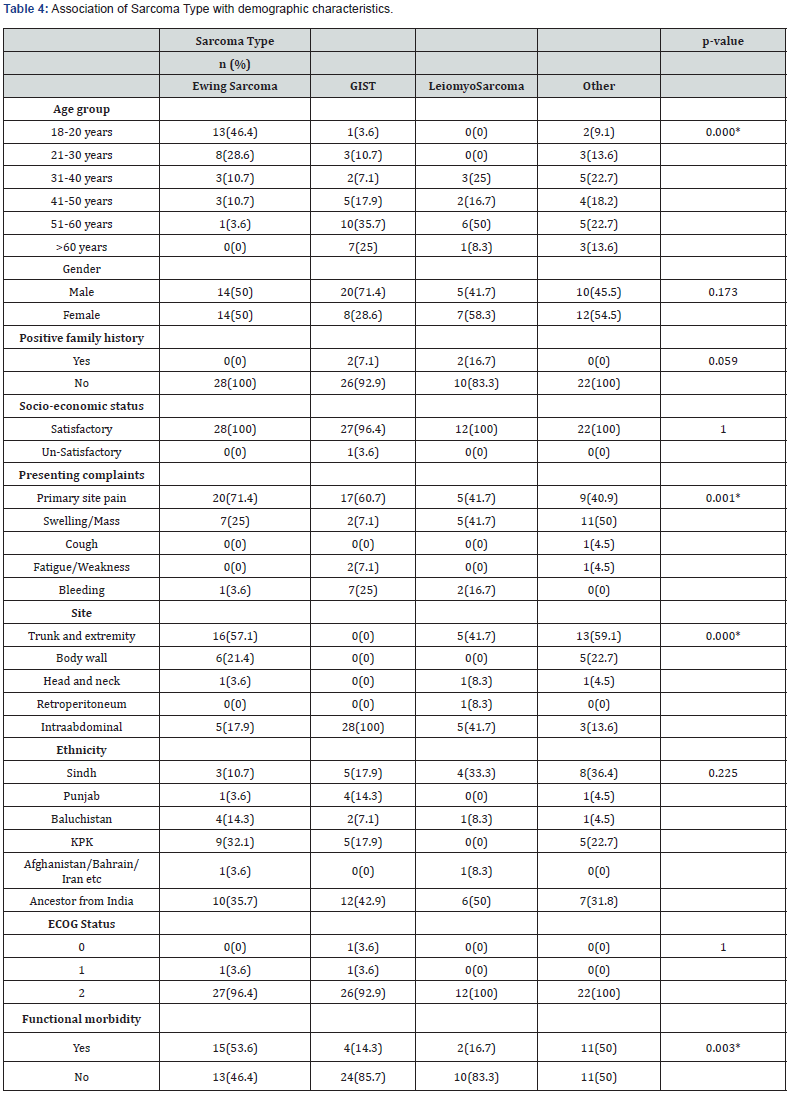
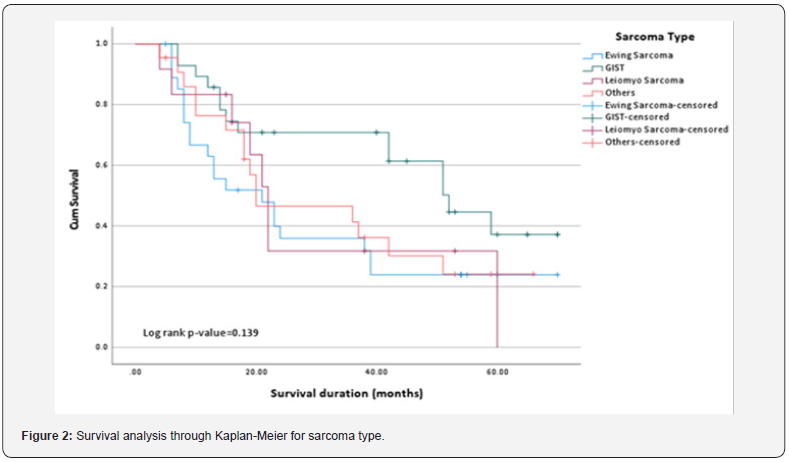
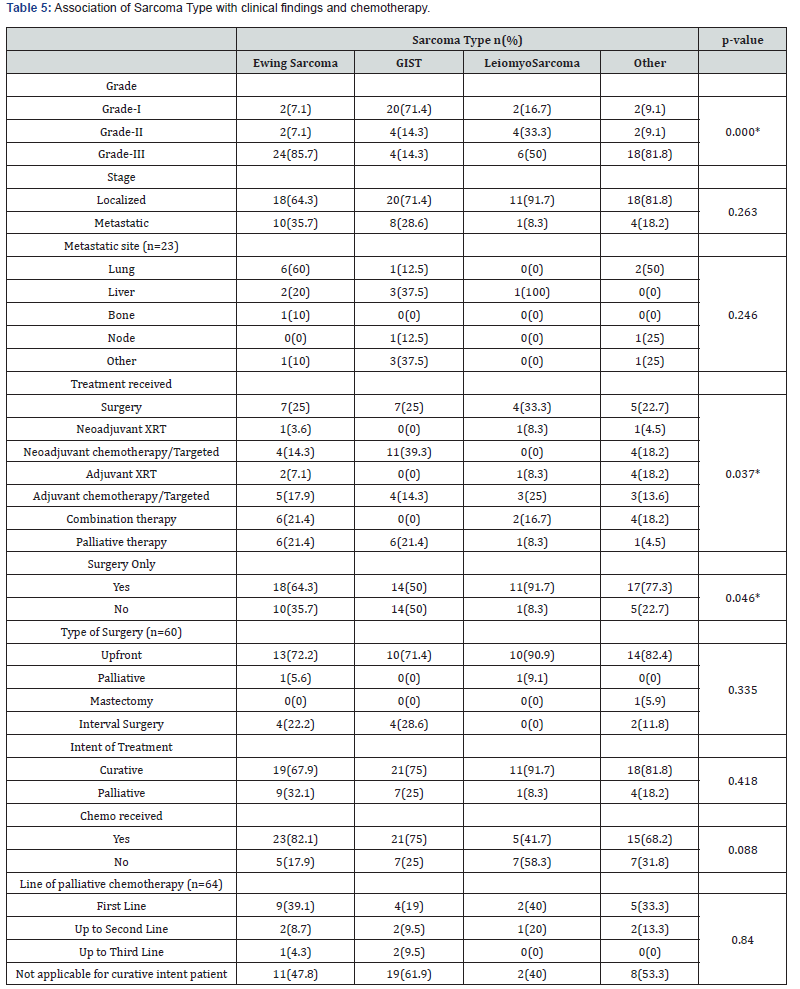
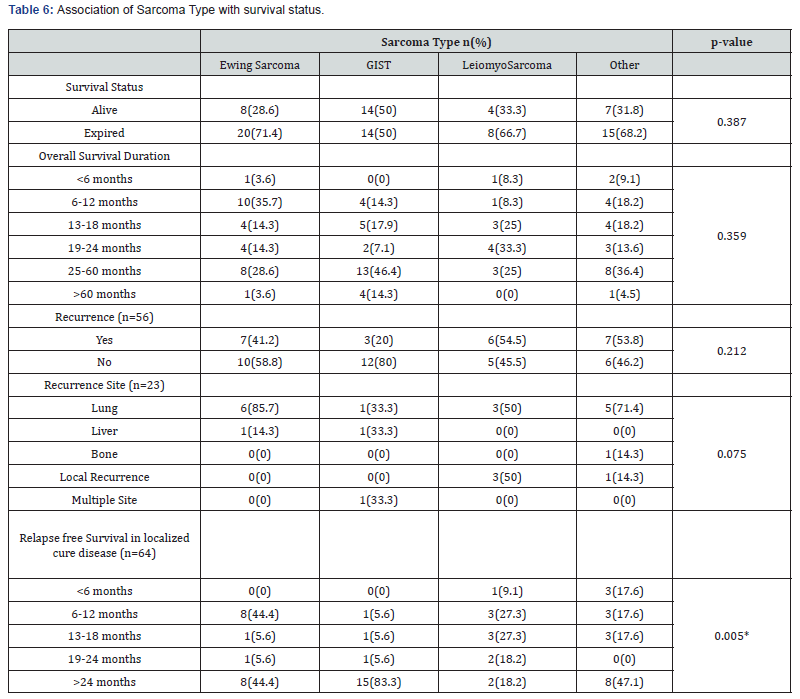
We also found the association of clinical characteristics with sarcoma type. The results in Table 5 and Table 6 showed that grade (0.000), treatment received (p=0.037), surgery alone (p=0.046), and relapse free survival in localized cure disease (p=0.005) were found to be significantly associated with the sarcoma type but we found stage (p=0.263), metastatic site (p=0.246), type of surgery (p=0.335), intent of treatment (p=0.418), chemo received (p=0.088), line of palliative chemotherapy (p=0.840), survival status (p=0.387), recurrence (p=0.212) and recurrence site (p=0.075) were insignificantly associated with type of sarcoma. The overall median survival time was observed as 24 months. Survival rate of different sarcoma types among patients was found as 50% for GIST, 33% for leiomyosarcoma, 28.6% for ewing sarcoma and 31% for another sarcoma. The survival distribution for the sarcoma type were statistically insignificant so we cannot conclude that the survival distributions are different in the population, (χ2=5.489, p=0.139). We observed that the patients who had GIST had longer survival time of 52 months as compared to leiomyosarcoma of 22 months, ewing sarcoma of 21 months and others of 20 months. Figure 2 shows the Kaplan-Meier survival analysis for sarcoma type.
Chi-square/fisher exact test was applied.
*Significant at 0.05 level.
Chi-square/fisher exact test was applied.
*Significant at 0.05 level.
Discussion
The diagnosis of soft tissue tumors is based on clinical, laboratory, imaging, and pathological findings. This approach is complex and requires a certain level of sophistication and expertise. Therefore, soft tissue sarcomas are sometimes misdiagnosed, resulting in unplanned resections or diagnostic delays. Although reports on the relationship between the additional wide excision after unplanned excision and local recurrence of soft tissue sarcomas are controversial, unplanned excision is generally considered to be associated with an increased risk of local recurrence and the needed for additional and more extensive surgery. A delay in diagnosing sarcoma will inevitably lead to tumor enlargement and large tumor size is a very poor prognostic factor. Successful prediction of soft tissue sarcoma using simple clinical characteristics and laboratory data will help in preventing misdiagnosis and improve the patient’s prognosis.14 In a study, ascertainment of the particular histologic subtype was performed through the means of image-guided percutaneous core needle biopsy, preferably with a co-axial technique to minimize the risk of seeding [19].
In a study mean age of participants 26.9±10.4 years and a median of 26 ranging from 10 years to 52 years. Ten participants were males and eight were females [19]. Studies also showed that STS occur at a much younger age (39.5 years) [20] as also reported for Western contries (64 years) [21]. In a study conducted in Pakistan [22] on 25 patients, among them 15 were males and 10 were females. In our study mean age of the participants was 41.44±16.71 years. The results have coincided with Liang et al [23].
In a study,1 finding regarding complaints of the participants shows that nearly 67% of the participants complain of swelling, three patients (16.7%) complain of pain, only two patients complain of ulceration and one participant discovered STS accidentally. The findings agreed with Kobayashi et al [24]. who found not one of their patients (n=14) had no history of trauma near where the tumor was located. In 14 cases, edema was the main issue, followed by pain (9 cases) and inflammation (2 cases), (7 cases). In the study that was conducted in Pakistan, the author reported a wound complication rate of 4%.22 In our study Primary site discomfort (56.7%), swelling/mass (27-8%), and bleeding (11.1%) were the most frequent presenting complaints.
The findings of a previous study1 reported that respecting sites of STS, seven patients (38.9%) had STS located in the thigh or buttock, three patients (16.7%) had STS located in the knee or lower leg, two patients (11.1%) had STS located in Arm. Three patients (16.7%) had STS located in the elbow or forearm. Three patients (16.7%) had located in the shoulder. The present findings agreed with Kobayashi et al [24] results showed that the thigh was the most common site for malignancies (7 cases), followed by the lower thigh (3), buttocks (2), knee (1), and the neck (1) (1 case each). In study results, 74.4% had a localized stage and 25.6% had a metastatic stage. The most frequent metastatic sites were liver 26.1% and lung 39.1%. Aliyu et al. [25] reported that 53 patients (43%) had primary soft tissue sarcoma in their extremities; of these, 41 (33%) had lesions in their lower extremities and 12 (10%) had involvement of their upper limbs. Twenty-five patients, or 20%, had involvement in their head and neck, followed by seventeen patients, or 14%, in their abdominal region, and ten patients, or 10%, in their thoracic region.
Regarding the characteristics of STS in the previous study results, the mean size was 20.1±10.6 cm, 10 patients (55.6%) had STS on the right side and 44.4% had STS on the left side. Only five patients (27.8%) had superficial STS and 13 (72.2%) had deep STS. The same study showed that 54.9% of tumors were smaller than 5 cm and 45.1% were larger than 5 cm, as previously reported by Liang et al.19 When broken down by body region, another study4 shows that 24.5% of tumors occurred in the upper extremities, 37.3% in the trunk/thoracic/abdominal wall, and 38.2% in the lower extremities. 44.2% were classified as having a surface tumor, whereas 58.8% were classified as having a deep tumor. Regarding the grades of STS, 5 (27.8%) were grade 1, 9 (50%) were grade 2, and 4 (22.2%) were grade 3.4 One study showed that regarding the stages of STS, 2 (11.1%) were stage IA, 3 (16.7%) were stage IB, 4 (22.2%) were stage IIA, 6 (33.3%) were stage IIB, 1 (5.6%) was stage IIIA and 2 (11.1%) were stage IIIB. Most of the patients were in stage II (B then A) followed by stage IB.
Regarding the histopathological diagnosis of STS, 2 (11.1%) had undifferentiated pleomorphic sarcoma, 3 (16.7%) had liposarcoma, 7 (38.9%) had rhabdomyosarcoma, 3 (16.7%) had myxofibrosarcoma, and 3 (16.7%) had leiomyosarcoma.1 The findings agreed with Liang et al. [5] who found that 28.7% of STS cases were fibrosarcoma, 13.2% were synovial sarcoma, 1.3% were alveolar soft part sarcoma and angiosarcoma, 6.5% were malignant peripheral neve sheath tumors, 2.9% were mesenchymal chondrosarcomas, and 2.5% were leiomyosarcomas. These results were in accordance with Kunhi et al [26] pleomorphic sarcoma was found to be the most common histologic subtype, accounting for 43% of all cases. Besides synovial sarcoma, liposarcoma (6.5%) and fibrosarcoma (4.7%) were also frequently encountered histologic categories (6.7 percent). The study conducted in Pakistan, liposarcoma was the most common histological type occurring in 24% of the patients. Other types included myxofibrosarcomas, synovial sarcoma and Leiomyosarcoma each reported in 12% patients. Other less common types included pleomorphic sarcoma, malignant peripheral nerve sheath tumour and rahabdomyosarcoma that were found in 8% patients [22].
In a series of 79 patients,16 with STS of extremities and trunk, overall survival (OS) is 77.6% and 3-year disease-free survival (DFS) is 74.3%. However, in another study, 3-year OS and DFS was not statistically different among grade II and grade III tumors [16]. It is possible to obtain reasonably good survival rates with careful pre-operative planning and appropriate and timely intervention. Recurrence of tumour remains an issue however. The use of adjuvant therapy in patients with limb sparing surgery for extremity STS has been widely accepted. Residual tumor is a risk factor for local recurrence, and it can be found in up to three-fourth of cases of unplanned resection as reported in many studies.49,50 Bianchi et al [27] reported that 5-year DSS was 77% in 452 patients with high-grade soft tissue sarcoma who received unplanned excision.
In series by Ogura et al., [28] tumor size and deep location greatly increase the risk of disease-specific death. Zhao et al. [29] also noted that larger tumor size (> 5 cm) is the only prognostic factor for OS. In our study, although OS at 3 years was not significantly different between the size groups (p=0.15) and the DFS at 3 years was significantly different between group 1 and group 2/3 (p=0.04) but was not significant between group 2 and group 3. In another study, 40% of the tumors with a high histologic grade were associated with recurrence or death. In contrast, a retrospective study observed that a high histologic grade was not associated with recurrence or poor overall survival [30]. Hassan et al. demonstrated an association between histologic subtype and overall survival, particularly outlining forbidding overall survival rates and outcomes portended by leiomyosarcomas [31].
In another study, the available clinicopathological characteristics of STS cohort stratified by sex revealed no significant differences apart from the tumor site, with the retroperitoneum more often involved in females, and the limbs or head and neck region in males. A possible explanation for this sex difference in primary sites might be that the limbs and the head and neck are most exposed to the above-mentioned occupational mutagens [9]. The small sample size of this study doe is a limitation for its applicability. It was conducted in urban environment; therefore, the results might not be generalized and applicable to larger populations.
Conclusion
The study highlighted the local demographics and outcomes of the rare diseases of STS. The outcomes are comparable to those of the developed world. Ewing sarcoma, leiomyosarcoma and GIST were the most frequently reported sarcomas however in our study population we have seen some very rare entities as well which further accentuate the diversity of the disease. Men are more likely to be diagnosed with soft tissue sarcoma than women, and middle-aged people are disproportionately afflicted. Rhabdomyosarcoma is the most prevalent histologic type, and the lower extremities are the most common location of involvement. The clinicopathological features of soft tissue sarcoma are the same regardless of the patient’s gender. Future studies should aim to explore novel therapeutic options and the role of genetic factors in STS to improve prognostic accuracy and treatment efficacy.
References
- Hamd M, Moursi A, Khalil A, Eltih O, Ashour H (2023) Study of Clinico-Pathological Characters of Extremity Soft Tissue Sarcoma. Egypt J Hosp Med 90(2): 2688-2694.
- Begum S, Kathirvelu S, Vaithy A, Srinivasan S (2019) Soft Tissue Sarcomas: An Overview on Histomorphology. Ann SBV 8(2): 45-50.
- Gamboa A, Gronchi A, Cardona K (2020) Soft tissue sarcoma in adults: an update on the current state of histiotype specific management in an era of personalized medicine. CA: A Cancer Journal for Clinicians 70(3): 200-229.
- Amankwah E, Conley A, Reed D (2013) Epidemiology and therapies for metastatic sarcoma. Clinic Epidemiol 5: 147-162.
- WHO Classification of Tumours Editorial Board eds. World health organization classification of soft tissue and bone tumours. 5th ed. Lyon: IARC Press (2020).
- Gamboa AC, Gronchi A, Cardona K (2020) Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin 70(3): 200-229.
- Buja A, Rugge M, Tropea S, Cozzolino C, Formaro CM, et al. (2023) Sex Differences in Soft Tissue Sarcoma: Incidence, Clinicopathological Profile, Survival, and Costs. J Womens Health (Larchmt) 32(11): 1257-1264.
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumour. 5th edition. International Agency for Research on Cancer 2020. WHO Classification of Tumours Series: Lyon; 2020.
- Gamboa AC, Gronchi A, Cardona K (2020) Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin 70(3): 200-229.
- Kolla´r A, Rothermundt C, Klenke Fl (2019) Incidence, mortality, and survival trends of soft tissue and bone sarcoma in Switzerland between 1996 and 2015. Cancer Epidemiol 63: 101596.
- Fujibuchi T, Miyawaki J, Kidani T, Imai H, Miura H (2020) Prediction of Soft Tissue Sarcoma from Clinical Characteristics and Laboratory Data. Cancers (Basel) 12(3): 679.
- Dangoor A, Seddon B, Gerrand C, Robert Grimer, Jeremy Whelan (2016) UK guidelines for the management of soft tissue sarcomas. Clini Sarco Res 6(1): 20.
- Kasraeian S, Allison D, Ahlmann E (2010) A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clini Orthopaed Related Research® 468(11): 2992-3002.
- Almas T, Khan M, Murad M, Muneeb Ullah, Adil Shafi, Maryam Ehtesham, et al. (2020) Clinical and Pathological Characteristics of Soft Tissue Sarcomas: A Retrospective Study from a Developing Country. Cureus 12(8): e9913.
- Hogg HDJ, Manas DM, Lee D, Dildey P, Scott J, Lunec J, et al. (2016) Surgical outcome and patterns of recurrence for retroperitoneal sarcoma at a single centre. Ann R Coll Surg Engl 98(3): 192-197.
- Garg L, Pruthi M, Batra U, Doval DC, Pasricha S, et al. (2022) Analysis of Clinical Outcomes of Patients with Soft Tissue Sarcoma. Ind J Surg Oncol 13(3): 518-524.
- Dapper H, Diehl C, Knebel C, Mogler C, Borm K, et al. (2023) Outcome of patients with soft tissue sarcomas of the extremities and trunk treated by (neo) adjuvant intensity modulated radiation therapy with curative intent. Radiat Oncol 18(1): 44.
- Von Mehren M (2020) NCCN guidelines insights: soft tissue sarcoma, version 12021. J Nat Comprehensive Cancer Net JNCCN 18(12): 1604-1612.
- Van Houdt WJ, Schrijver AM, Cohen-Hallaleh RB (2017) Needle tract seeding following core biopsies in retroperitoneal sarcoma. Eur J Surg Oncol 43(9): 1740-1745.
- Talati N, Pervez S (1998) Soft tissue sarcomas: pattern diagnosis or entity? J Pak Med Assoc 48(9): 272-275.
- Gustafson P (1994) STS epidemiology and prognosis in 508 patients. Acts Orthop Scand 259: 1-30.
- Umer M, Saeed J, Shamsi Z, Tariq MU (2021) Treatment and outcomes of soft tissue sarcoma of groin, hip and thigh: a retrospective review from a tertiary care hospital. J Pak Med Assoc 71(Suppl 5)(8): 75-78.
- Liang Y, Guo T, Hong D, Wei Xiao, Zhiwei Zhou (2020) Time to local recurrence as a predictor of survival in patients with soft tissue sarcoma of the extremity and abdominothoracic wall. Frontiers in Oncology 10: 599097.
- Kobayashi H, Ae K, Tanizawa T, Tabu Gokita, Noriko Motoi (2015) A Clinicopathological Analysis of Soft Tissue Sarcoma with Telangiectatic Changes. Sarcoma 2015: 740571.
- Aliyu U, Okuofo E, Okwonna C (2016) Clinicopathological pattern of soft tissue sarcoma in a tertiary health institution in Northwestern Nigeria. Int J Res in Med Sc 6(5): 1632-1638.
- Kunhi M, Pradhan S, Bhattacharyya S (2020) Clinicopathological study of soft tissue sarcoma-retrospective study of tertiary cancer institute in eastern India. Int J Res Med Sci 8(11): 4075-4078.
- Bianchi G, Sambri A, Cammelli S, A Galuppi, A Cortesi (2017) Impact of residual disease after ‘unplanned excision’ of primary localized adult soft tissue sarcoma of the extremities: evaluation of 452 cases at a single institution. Musculoskelet Surg 101(3): 243-248.
- Ogura K, Higashi T, Kawai A (2017) Statistics of soft-tissue sarcoma in Japan: report from bone and soft tissue tumor registry in Japan. J Orthop Sci 22(4): 755-764.
- Zhao R, Yu X, Feng Y, Wang J, Mao Y, Yin W et al. (2018) The influence of anatomoic location on outcomes in patients with localized primary soft tissue sarcoma. Jpn J Clin Oncol 48(9): 799-805.
- Garcia-Ortega DY, Villa-Zepeda O, Martinez-Said H, Cuellar-Hübbe M, Luna-Ortiz K (2016) Oncology outcomes in retroperitoneal sarcomas: prognostic factors in a retrospective cohort study. Int J Surg 32: 45-49.
- Hassan I, Park SZ, Donohue JH, David M Nagorney, Paul A Kay, et al. (2004) Operative management of primary retroperitoneal sarcomas: a reappraisal of an institutional experience. Ann Surg 239(2): 244-250.






























