Role of FDG PET /CT in Detection of Primary Tumors in Patients with Bone Metastasis of Unknown Origin
Maie El Saied Mohamed Sabae*, Ahmed Farid Youssef, Mohamed Fathy Ragab, Hamada Mohamed Khater
Faculty of medicine, Benha university, Egypt
Submission: April 18, 2024; Published: May 17, 2024
*Corresponding Address: Maie El Saied Mohamed Sabae, Faculty of medicine, Benha university, 28st, Mahalla el kobra, Gharbia, Egypt
How to cite this article: Maie El Saied Mohamed Sabae*, Ahmed Farid Youssef, Mohamed Fathy Ragab, Hamada Mohamed Khater. Role of FDG PET /CT in Detection of Primary Tumors in Patients with Bone Metastasis of Unknown Origin. Canc Therapy & Oncol Int J. 2024; 27(1): 556201. DOI:10.19080/CTOIJ.2024.27.556201
Abstract
Background: To evaluate the effectiveness of FDG-PET-CT in identifying the initial tumor in individuals with bone metastases from an unidentified source.
Methods: The study is designed as a prospective case control study. Data will be obtained from prospective 50 patients admitted to the oncology department of El Mobara hospital of medical insurance or presented to its outpatients’ clinic. The study will be conducted in the PET-CT Unit in (Life Scan Center) and (PROF DR. Khaled Dewan Center).
Results: The study included 50 patients with higher male predominance (68%). Most of patients aged above 40 years old (76%). Most of the lesions were osteolytic lesions (80%) while 30% were sclerotic and 16% were mixed. Most of lesions were multifocal (66%) while 32% were focal lesions and 4% were diffuse. Minimum SUV of the bony lesions ranged from 0 to 13 with median 6 and maximum SUV ranged from 0 to 35 with median 12. The PET scan was positive for 42 lesions and negative for 8 lesions. Out of 42 positive PET lesions, primary lesions outside the bone were detected in 30 patients while 12 lesions were positive to be primary lesions in the bone. The primary lesions as detected by bone scan were prostate in 9 cases, multiple myeloma in 7 cases, breast in 5 cases, bone in 5 cases, pulmonary in sex cases, adrenal in 3 cases, renal in 2 cases, hepatic in 2 cases and pancreas, pleural or sarcoma in 1 case.
Conclusions: PET CT is the best imaging modality and is the modality of choice for optimal detection of primary tumors in cases of osseous bone metastasis of unknown primary.
Keywords: FDG; PET; CT; Bone metastasis; Unknown primary tumors
Abbreviations: BMUO: Bone Metastasis of Unknown Origin; CUP: Cancer of Unknown Primary; PET: Positron Emission Tomography; CT: Computed Tomography; FDG: Floro-Deoxy Glucose; MUO: Metastasis of Unknown Origin; N: Number; MM: Multiple Myeloma; Max: Maximum; SUV: Standard Uptake Value; MIP: Maximum Intensity Protections; IV: Intra Venous; 3D: Three Dimensions; Mets: Metastasis
Background
Bone metastasis is the predominant location for the spread of cancers of unknown origin (MUO), with about 40% of these patients experiencing bone-related symptoms. Identifying the original cancer site in such instances is crucial for determining the stage, formulating an effective treatment plan, and forecasting the patient’s outcome [1]. Unlike traditional imaging techniques, F18-floro-deoxy glucose PET/CT ([18F] FDG-PET/CT) capitalizes on the heightened glucose metabolism found in many cancers (known as the Warburg effect) to identify unusual [18F] FDG absorption, thereby presenting no significant limitations [2].
For over twenty years, [18F] FDG-PET/CT has been recommended for locating hidden primary tumors that remain undetectable through standard diagnostic approaches, especially in patients with bone metastases from an unidentified source (BMUO) [3]. It is recommended as a highly sensitive and valuable diagnostic tool, potentially serving as the primary method for identifying BMUO [4]. The integration of 18-fluorodeoxyglucose positron emission tomography (FDG-PET) with computed tomography (CT) forms a critical diagnostic instrument in clinical oncology, offering the benefits of both metabolic and anatomical imaging [5].
Utilizing PET/CT as the initial step in patient evaluation could lower expenses, save time, and direct subsequent tests and biopsies. Furthermore, replacing traditional imaging with [18F] FDG-PET/CT could result in the earlier identification of the primary tumor, thereby allowing for the commencement of targeted treatment sooner [6]. FDG-PET/CT proves beneficial in differentiating between cases presenting with FDG-positive bone lesions without evidence of FDG activity outside the skeleton. Additionally, bone abnormalities stemming from non-cancerous conditions often do not show FDG uptake and typically exhibit CT characteristics indicative of bone injury, SAPHO syndrome, or age-related changes [7]. Although PET/CT scans utilize the increased glucose consumption of cancer cells, FDG PET/CT cannot exclusively distinguish malignant growths, as it might also highlight inflammatory conditions and certain non-cancerous tumors [8].
Research Objective
The purpose of this study is to explore the effectiveness of FDG-PET-CT in the prompt identification of the originating tumor in individuals with bone metastases of unidentified primary origin.
Methods
Patients
PETCT of 50 patients with bone lesions, were required from the outpatient oncology clinic at the Mobara hospital from March 2022 to January 2024 clinically suspected of having primary malignancy. Thirty-four of them were males and twenty-six were females with age group from 6 to 70 years. Nineteen of them with unilateral and one bilateral diabetic foot, adult patients (n=48) and one child (n=2).
Examinations
Every participant underwent a PET-CT scan using a specialized scanner. Participants were required to fast for a minimum of 6 hours prior to the administration of FDG, with a pre-scan fasting blood glucose level under 150 mg/dl being mandatory for all. Scans commenced 1 hour following the intravenous injection of FDG, with dosages ranging from 0.07 to 0.1 mCi/kg, during which time patients were asked to remain at rest. The CT scan covered the area from the base of the skull to the mid-thigh, with no oral contrast utilized and only water employed to clarify the imaging of the bowel. For those with normal kidney function and no known allergies to IV contrast agents, a dose of 100-130 ml of omnipaque (containing 300 mg of iodine per ml) was administered. The PET emission scans took 2 minutes per bed position, leading to total scan durations of between 15 and 20 minutes per patient. The combined CT and PET images were then analyzed on a workstation in axial, coronal, sagittal, and 3D maximum intensity projection (MIP) views.
Ethical Considerations
Informed consent, both written and verbal, will be secured from each participant, ensuring their confidentiality and privacy are fully protected. The data collected will solely be used for the purposes of this research and will not be repurposed for any other use.
Results
The study included 50 patients with higher male predominance (68%). Most of patients aged above 40 years old (76%). Most of the lesions were osteolytic lesions (80%) while 30% were sclerotic and 16% were mixed. Most of lesions were multifocal (66%) while 32% were focal lesions and 4% were diffuse. Minimum SUV of the bony lesions ranged from 2 to 13 with median 5 and maximum SUV ranged from 2 to 35 with median 11. The PET scan was positive for 42 lesions and negative for 8 lesions. Out of 42 positive PET lesions, primary lesions outside the bone were detected in 30 patients while 12 lesions were positive to be primary lesions in the bone. The primary lesions as detected by bone scan were prostate in 9 cases, multiple myeloma in 7 cases, breast in 5 cases, bone in 5 cases, pulmonary in 6 cases, adrenal in 3 cases, renal in 2 cases, hepatic in 2 cases and pancreas, pleural or sarcoma in 1 case PET scan showed presence of extraosseous lesions in 33 cases. Lymph nodes were affected in 21 patients. Out of them, 17 lesions were extended to other organs than lymph nodes (1 kidney, 3 liver, 2 lung, and 9 liver and lung). 12 extraosseous lesions were detected without lymph node affection (4 hepatic lesions, 2 lung, 1 soft tissue, 5 liver and lung). A biopsy was performed for 48 lesions. Extra osseus primary lesions were present in 31 patients (62%). Primary bone malignant bone lesions were detected in 11 patients distributed as follows: 7 MM, 3 B cell lymphoma and 1 Ewing sarcoma.
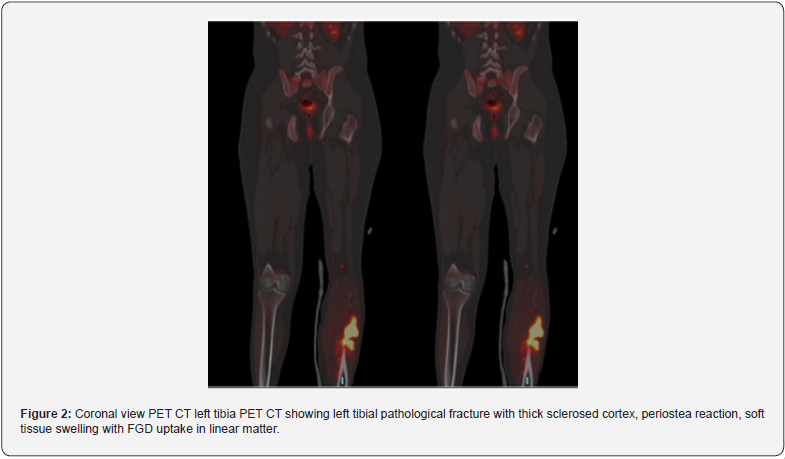
Primary osseus malignant lesions were detected in 11 patients (22%) (Figure 1) and osseous benign lesions were detected in 6 patients (12%) (Figure 2). Out of 31 extra osseus malignant primary lesions, 9 was prostatic acinar adenocarcinoma, 4 invasive ductal carcinoma of the breast, 1 mastitis carcinomatosis, 5 pulmonary adenocarcinoma, 1 squamous cell carcinoma of the lung, 2 adrenocortical carcinoma, 1 papillary and 2 clear cell renal carcinoma, 2 hepatocellular carcinoma, 1 epithelial cell carcinoma of the ovary, 1 undifferentiated pleomorphic lesions of sarcoma and 1 mesothelioma of the pleura (Figures 1 & 2).
Discussion
The occurrence of bone metastases in individuals with cancer significantly impacts both survival rates and quality of life, leading to complications such as pain, fractures, elevated blood calcium levels, and suppression of bone marrow. This underscores the critical importance of prompt and precise identification of bone metastases [9].

In the study under consideration, a cohort of 50 patients with metastatic bone lesions of unknown primary origin was examined, with a predominant majority being male (68%). This finding aligns with the research conducted by Huey et al., (2021), which reported that 52% of individuals with bone metastases of indeterminate primary were male. Similarly, Budak & Yanarates (2020) observed in their analysis of 100 patients with unknown primary bone metastases that males constituted 74% of the study population. Furthermore, Li et al., [9] also noted a male predominance (63.7%) in their larger study of 344 patients with bone metastases. Park et al., [10] showed higher male predominance among bony metastasis patients (66%). Also, in Xu, (2009) most of included patients with osteolytic lesions were males (56.3%). On the other hand, Soni et al., [11] showed comparable sex distribution in patients with bony metastasis.
Most of the included patients aged above 40 years in the present study.
Similarly, Soni et al., [11] showed that mean age of patients with bony metastasis was 59.3 ± 14 years. Similarly, Huey et al., (2021) in his study on 29 bone lesions with unknown primary patients reported that all patients aged above 60 years. In a study by Budak & Yanarates, (2020), the patients had a mean age of 61 ± 3.2 years. Mean age of patients with bony metastasis with unknown primary was 58.2 ± 15.6 years in a study by Li et al., [9]. In Park et al., [10], mean age for patients with metastatic bony lesions was 60 ± 13. Xu, (2009) showed that the mean age for patients with osteolytic lesions was 61.4 ± 13.5 years. Most of bony lesions were of osteolytic type (80%) while sclerotic lesions represented 30% of lesions. In concordance with the present study, Li et al., (2015) showed that most of bony metastatic lesions were osteolytic lesions (69.3%). Raphael et al., (2013) showed that 50% of bone lesions of unknown primary were lytic lesions.
In the present study, most of lesions were multifocal (66%) and 32% were focal lesions. In agreement with the present study, Budak & Yanarates, (2020) showed that 90% of bony metastatic lesions were multifocal. Soni et al., [11] also in his study on 83 patients showed that 85.5% of lesions were multifocal. In Park et al., [10], most of lesions were diffuse multifocal. In the present study, median SUV min was 5 and ranged from 2 to 13 while SUV max was 11 and ranged from 2 to 35. In agreement with the present study, Soni et al., [11] reported that median SUV max for metastatic osteolytic lesions was 9.9. Li et al., [9] who evaluated 8612 bony lesions showed that median SUV min of metastatic lesions was 5.5. Cengiz et al., (2018) reported median SUV max equal to 11.5 for metastatic bony lesions. Xu, (2009) reported median SUV max 12.6 for patients with metastatic bony lesions. On contrary, lower median SUV max was reported in another study. Li et al., (2015) also reported median SUV max 8.5 for metastatic bony lesions. Riaz et al., [12] showed that the average SUV max value of the hypermetabolic lesions was reported to be 8.2 ± 4.7.
Accuracy of PET CT in detection of primary lesions:
Regarding our findings, PET scan was positive for malignant bone lesions in 84% of patients. Out of which, 41 were true positive representing malignant bone lesions (either primary or Mets) and 1 lesion was false positive. PET scan showed negative results in 8 patients, out of which 6 were true negative (benign bony lesions) and 2 were false negative. Thus, PET scan had 95.3% sensitivity and 85.7% specificity in detection of the malignant bone lesions.
Consistent with the findings of this study, Budak & Yanarates (2020) demonstrated that PET/CT is a preferred initial diagnostic tool for identifying the primary source in patients with bone metastasis of unknown origin (BMUO), recording sensitivity, specificity, accuracy, and detection rates of 84.7%, 46%, 79%, and 72%, respectively. Other studies assessing the efficacy of PET/CT in locating primary tumors in patients with unknown primary bone metastases have found similar sensitivity rates but reported greater specificity compared to this study. The relatively lower specificity observed in this study might be attributed to a significant proportion of patients having prostate cancer, which is known for the minimal FDG uptake by prostatic lesions limited to the organ.
Cengiz et al., (2018) reported that in their research, primary tumors were accurately identified in 59 out of 121 patients (49%) using 18F-FDG PET/CT whole-body imaging, with sensitivity, specificity, and accuracy rates of 84%, 78%, and 82%, respectively. Furthermore, in research involving 75 patients with BMUO, the primary cancer was found in 61 patients, with PET/ CT yielding true positive results in 46 cases. This underscores the effectiveness of PET/CT as an instrumental approach in pinpointing the primary cancer [10].
Han et al., (2012) endorse PET/CT’s elevated sensitivity for identifying the primary tumor in patients with cancer of unknown primary (CUP), recommending its use as a primary diagnostic method. They reported sensitivity, specificity, and accuracy rates of 91.5%, 85.2%, and 88.3%, respectively, in a study involving 120 patients with unknown primary bone metastasis. Riaz et al., [12] found that PET/CT’s effectiveness in identifying primary cancer in a cohort of 82 patients with unknown primary bone metastases was characterized by sensitivity, specificity, and accuracy rates of 80%, 74%, and 78%, respectively. Yanagawa et al., (2010) explored PET/CT’s utility in locating the primary tumor in patients with metastases to bone and soft tissues. Out of 71 patients in their study, 24 underwent PET/CT scans, achieving a 50% detection rate (12 out of 24 patients), with no marked difference in sensitivity observed when compared to traditional imaging techniques. Roh et al., [13] reported similar findings in their study.
Conversely, some earlier research has documented a reduced sensitivity and specificity for PET scans in identifying malignant bone lesions. Shimada et al. [14] observed a detection rate of only 43% (17 out of 39) in a study focusing on patients with bone metastasis of unknown origin (BMUO), finding no significant difference in performance compared to CT scans. Takagi et al., [15] managed to detect the primary tumor in 30 out of 80 BMUO patients using PET scans, including identifying the primary lesion in three out of 21 patients where other methods had failed.
Conclusion
In summary, these findings highlight PET CT as a highly sensitive and accurate tool for the identification of primary tumors in patients with unknown primary bone metastases.
References
- Varadhachary G, Abbruzzese JL (2020) Carcinoma of unknown primary. Abeloff’s Clin Oncol 1694-1702.
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA. Cancer J Clin 69(1): 7-34.
- Maghami E (2020) Diagnosis and management of squamous cell carcinoma of unknown primary in the head and neck: ASCO guideline. J Clin Oncol 38(22): 2570-2596.
- Park SB, Park JM, Moon SH, Cho YS, Sun JM, et al. (2018) Role of18F -FDG PET/CT in patients without known primary malignancy with skeletal lesions suspicious for cancer metastasis. PLoS One 13(5): e0196808.
- Stalder SA, Schumann P, Lanzer M (2020) Value of SUV. Biology (Basel) 9(2): 23.
- Huang J, Heng J, Zhang W, Liu Y, Xia T, et al. (2022) Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. Sem Cell & Dev Biol 128: 137-144.
- Ben-Him S, Ell P (2019) 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med 50(1): 88-99.
- Cengiz A, Göksel S, Yürekli Y (2019) Diagnostic value of 18F-FDG PET/CT inpatients with carcinoma of unknown primary. Mol Imaging Radionucl Ther 27(3): 126-132.
- Li X, Wu N, Zhang W, Liu Y, Ming Y (2020) Differential diagnostic value of 18F-FDG PET/CT in osteolytic lesions. J Bone Oncol 24: 100302.
- Park SB, Park JM, Moon SH, Cho YS, Sun JM, et al. (2018) Role of 18F-FDG PET/CT in patients without known primary malignancy with skeletal lesions suspicious for cancer metastasis. PLoS One 13(5): e0196808.
- Soni N, Ora M, Aher PY, Mishra P, Maheshwarappa RP, et al. (2021) Role of FDG PET/CT for detection of primary tumor in patients with extra cervical metastases from carcinoma of unknown primary. Clin Imaging 78: 262-270.
- Riaz S, Nawaz MK, Faruqui ZS, Kazmi SAS, Loya A, et al. (2016) Diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography-computed tomography in the evaluation of carcinoma of unknown primary. Mol Imaging and Radionucl Therap 25(1): 11-18.
- Roh JL, Kim JS, Lee JH, Cho KJ, Choi SH, et al. (2009) Utility of combined 18F-fluorodeoxyglucose-positron emission tomography and computed tomography in patients with cervical metastases from unknown primary tumors. Oral Oncol 45(3): 218-224.
- Shimada H, Setoguchi T, Yokouchi M, Sasaki H, Ishidou Y, et al. (2014) Metastatic bone tumors: Analysis of factors affecting prognosis and efficacy of CT and 18F FDG PET CT in identifying primary lesions. Mol Clin Oncol 2(5): 875-881.
- Takagi T, Katagiri H, Kim Y, Suehara Y, Kubota D, et al. (2015) Skeletal metastasis of unknown primary origin at the initial visit: a retrospective analysis of 286 cases. PluS one 10(6): e0129428.






























