The Importance of Developing Cell Penetrating Antibodies for Cancer Therapy
Sunanda Singh*, Shivanshu Kumar, Hector J Gomez and Ashutosh S Parihar
Singh Biotechnology, 1547 Fox Grape Loop, Florida, USA
Submission: March 04, 2024; Published: March 11, 2024
*Corresponding Address: Sunanda Singh, Singh Biotechnology, 1547 Fox Grape Loop, Lutz, Florida, 33558, USA, Email: ssingh@singhbiotechnology.com
How to cite this article: Sunanda Singh*, Shivanshu Kumar, Hector J Gomez and Ashutosh S Parihar. The Importance of Developing Cell Penetrating Antibodies for Cancer Therapy. Canc Therapy & Oncol Int J. 2024; 26(2): 556184. DOI:10.19080/CTOIJ.2024.26.556184
Opinion
It is believed that most of the important druggable targets in medicine are located inside the cell [1]. Some of the most sought-after high-profile targets include RAS, RAF, MEK, ERK, PI3K, p38, mTOR, JAK, STAT3, STAT5, MYC, and many others. Developing small molecule inhibitors has several drawbacks. Some of these include difficulties in developing robust screening assays and high throughput screens to find potential candidates, the potential toxicity associated with small molecules and its breakdown products by the liver, and the development of acquired drug resistance (ADR). Therapeutic antibodies have revolutionized drug development. Once a molecule has been identified as a key factor in the pathogenesis of a disease, then this human protein can be used to immunize a mouse to generate antibodies for this target protein. Using screening processes, the cDNA of the antibody can be identified. It then will be humanized and tested for safety, tolerability, and efficacy. This very simplified description has led to the development of multiple antibody-based drugs such as bevacizumab, trastuzumab, rituximab, ipilimumab, nivolumab, pembrolizumab, and many others.
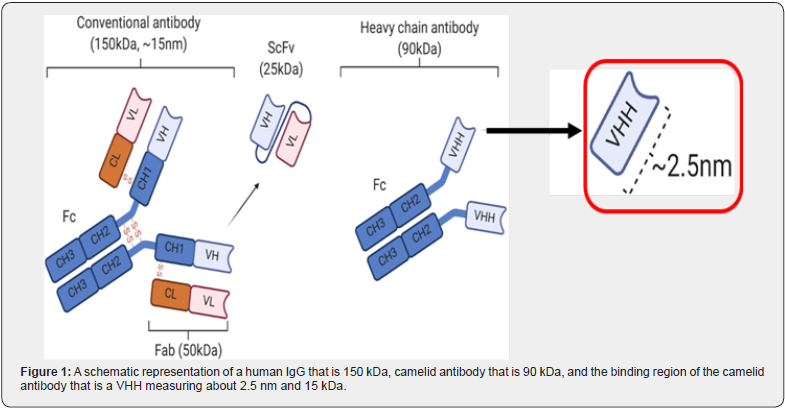
Unfortunately, these therapeutic monoclonal antibodies are approximately 150 kDa in size and are too large to cross the cell membrane. Thus, they are developed to target extracellular proteins in the blood, extracellular space, or receptors on the surface of the cell membrane. The discovery of camelid antibodies (heavy chain only and devoid of light chains) [2] and the further discovery that its binding region, the VHH, can function as an independent nano-antibody by Raymond Hamers was significant [3]. The VHH is approximately 15kDa or 1/10th the size of a human IgG (Figure 1). This small size along with other inherent properties of the VHH nano-antibody are a promising starting point for developing cell penetrating antibodies.
A myriad of attempts has been made to create cell penetrating antibodies. An anti-GFAP VHH was developed by Pierre Lafaye’s group that crossed the blood-brain barrier (BBB) and was intended for diagnostic purposes [4]. A full-length IgG was developed by Orum Therapeutics to target RAS by crossing the cell membrane [5,6]. Additionally, Hua Yu and her group developed an anti-STAT3 acetylated peptide which facilitated cell membrane penetration [7]. Unfortunately, none of these cell penetrating antibody technologies have reached clinical trials yet.
A series of cell penetrating nano-antibodies based on the VHH platform have been developed to target intracellular KRAS and STAT3. These are:
i. SBT-100 which is bifunctional and binds to both KRAS and STAT3,
ii. SBT-101 which only binds to STAT3, and
iii. SBT-102 which only binds to KRAS [8,9].
All three of these nano-antibodies bind to their targets with nano-molar affinity and thus inhibit the growth of human cancers in vitro. SBT-100 has undergone much more testing and preclinical development than SBT-101 and SBT-102. SBT-100 inhibits the GTPase activity of KRAS and down-regulates p-ERK in human cancer with a KRAS(G13D) mutation. With a pancreatic cancer (PANC-1) having KRAS(G12D) and triple negative breast cancer (TNBC) (MDA-MB-231) having KRAS(G13D) mutation SBT-100 gave a dose response growth suppression of 85% and 89%, respectively, in a 72-hour MTT assay. SBT-100 gives significant growth suppression against 11 solid human cancers (2 pancreatic cancers, 3 TNBCs, ER+PR+ breast cancer, HER-2 amplified breast cancer, glioblastoma, 2 sarcomas, and metastatic, chemo-resistant prostate cancer), and 2 leukemias. In vivo, both in a xenograft mouse model during a 3-week study [9] the MDA-MB-231 tumors were treated with SBT-100 as mono-therapy; and the PANC-1 tumors received either gemcitabine only, SBT-100 only, and combination therapy of gemcitabine plus SBT-100. All three groups had significant suppression of the PANC-1 tumors after 3 weeks, but combination therapy doubled the suppression seen with gemcitabine alone. Monotherapy of SBT-100 against the MDA-MB-231 tumors gave significant growth suppression too.
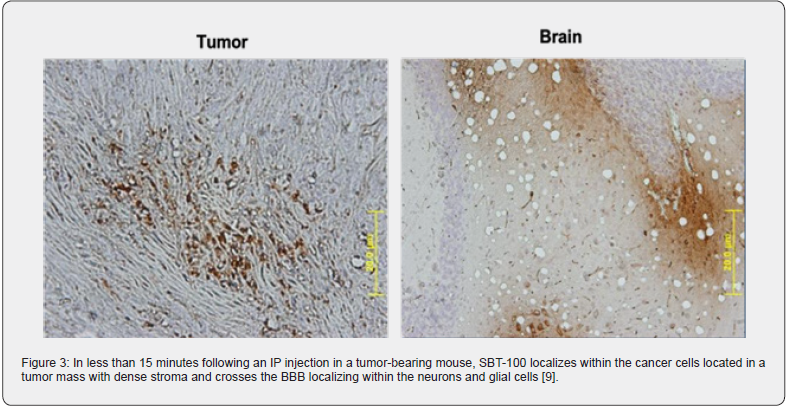
SBT-100 penetrates the cell membrane (Figure 2) and BBB very rapidly in vivo. When a tumor-bearing xenograft mouse is injected intraperitoneally with SBT-100 and then sacrificed 15 minutes later, immunohistochemical staining reveals the presence of SBT-100 inside the cancer cells within the tumor mass with dense stroma and inside the neuron and glial cells of the brain [9] (Figure 3). Being able to cross the BBB so quickly is a major potential benefit in treating cancer patients with primary CNS malignancies or metastatic cancers that travel to the CNS. SBT- 100’s ability to cross the BBB is consistent with the work done by Li et al. [10]. Furthermore, in a non-malignant animal model, autoimmune uveitis of the eye, revealed that SBT-100 crosses the blood retina barrier (BRB) to give a therapeutic effect in the treatment of autoimmune uveitis by targeting STAT3 [11]. By targeting STAT3 and inhibiting its effects, SBT-100 has given a therapeutic response in two other autoimmune disease models and a neurodegenerative disease model (unpublished data).

Monomeric VHH molecules have a 1-2-hour serum half-life, and this is also the case for SBT-100. This very short half-life helps to minimize toxicity. In addition, the camelid VHH is >90% homologous with the human VH. Thus, this reduces the potential for ADA (anti-drug antibody) formation. VHHs has excellent stability and solubility. Because VHHs do not generally aggregate, this further reduces the risk of ADA formation. Currently, there are two VHH drugs, caplacizumab and ozoralizumab, in the market with no major safety issues. Also, several other VHH drugs have gone through Phase 1, 2, and 3 without major toxicity problems. To date approximately 400 animals have been treated with SBT- 100 without weight loss, toxicity, or death.
Using the VHH nano-antibody platform seems to be a promising approach for the development of cell penetrating antibodies for cancer therapy. Since most of the cancer promoting and causing proteins are intracellular, this further emphasizes the importance of developing these cell penetrating antibodies not just targeting the primary tumor but also metastases that travel to the brain. This approach represents a major paradigm shift in drug development.
References
- Gordon RE, Nemeth JF, Singh S, Lingham RB, Grewal IS (2021) Harnessing SLE Autoantibodies for Intracellular Delivery of Biologic Therapeutics. Trends Biotechnol 39(3): 298–310.
- Arbabi Ghahroudi M, Desmyter A, Wyns L, Hamers R, Muyldermans S (1997) Selection and identification of single domain antibody fragments from camel heavy‐chain antibodies. FEBS Lett 414(3): 521-52
- Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hammers C, et al. (1993) Naturally occurring antibodies devoid of light chains. Nature 363(6428): 446–448.
- Perruchini C, Pecorari F, Bourgeois JP, Duyckaerts C, Rougeon F, et al. (2009) Llama VHH antibody fragments against GFAP: better diffusion in fixed tissues than classical monoclonal antibodies. Acta Neuropathol 118(5): 685-695.
- Choi DK, Bae J, Shin SM, Shin JY, Kim S, et al. (2014) A general strategy for generating intact, full-length IgG antibodies that penetrate into the cytosol of living cells. mAbs 6(6): 1402-1414.
- Shin SM, Choi DK, Jung K, Bae J, Kim JS, et al. (2017) Antibody targeting intracellular oncogenic Ras mutants exerts anti-tumour affects after systemic administration. Nature Communications 8: 15090.
- Aftabizadeh M, Li YJ, Zhao Q, Zhang C, Ambaye N, et al. (2021) Patent antitumor effects of cell-penetrating peptides targeting STAT3 axis. JCI insight 6(12): e136176.
- Singh S, Murillo G, Chen D, Parihar AS, Mehta RG (2018) Suppression of Breast Cancer Cell Proliferation by Selective Single-Domain Antibody for Intracellular STAT3. Breast Cancer (Auckl) 3:12:1178223417750858.
- Singh S, Murillo G, Richner J, Singh SP, Berleth E, et al. (2022) A Broad-Based Characterization of a Cell-Penetrating, Single Domain Camelid Bi-Specific Antibody Monomer That Targets STAT3 and KRAS Dependent Cancers. Int J Mol Sci 23(14): 7565.
- Li T, Bourgeois J, Celli S, Glacial F, Le Sourd A, et al. (2012) Cell‐penetrating anti‐GFAP VHH and corresponding fluorescent fusion protein VHH‐GFP spontaneously cross the blood‐brain barrier and specifically recognize astrocytes: application to brain imaging. The FASEB Journal 26(10): 3969–36
- Mbanefo EC, Yan M, Kang M, Alhakeem SA, Jittayasothorn Y, et al. (2021) STAT3-Specific Single Domain Nanobody Inhibits Expansion of Pathogenic Th17 Responses and Suppresses Uveitis in Mice. Front Immunol 15: 12.






























