Ketogenic Diet Approaches in High Grade Gliomas: An Update on Preclinical and Clinical Data
Andrea Salmaggi1, Annachiara D’Urso2, Francesco Maria Guida3, Annica Piccardi⁴, Antonio Silvani⁵ and Emilio Ciusani2*
1Department of Neurosciences, ASST Lecco, Lecco, Italy
2Department of Diagnostic and Technology, Fondazione IRCCS Istituto Neurologico C Besta, Milan, Italy
3Department of Oncology, ASST Lecco, Lecco, Italy
4Department of Neuro-oncology, Fondazione IRCCS Istituto Neurologico C Besta, Milan, Italy
5Department of Neuro-oncology, Fondazione IRCCS Istituto Neurologico C Besta, Milan, Italy
Submission: February 19, 2024; Published: March 05, 2024
*Corresponding Address: Emilio Ciusani, PhD, Department of Diagnostic and Technology, Fondazione IRCCS Istituto Neurologico Carlo Besta, via Celoria 11, Milano, 20133, Italy, Email: emilio.ciusani@istituto-besta.it
How to cite this article: Andrea Salmaggi, Annachiara D’Urso, Francesco Maria Guida, Annica Piccardi, Antonio Silvani and Emilio Ciusani*. Ketogenic Diet Approaches in High Grade Gliomas: An Update on Preclinical and Clinical Data. Canc Therapy & Oncol Int J. 2024; 26(2): 556182. DOI:10.19080/CTOIJ.2024.26.556182
Abstract
Due to its aggressive nature and resistance to conventional therapies, Glioblastoma (GBM) poses a significant challenge. Despite decades of research, prognosis remains poor, necessitating innovative treatment strategies. Metabolic dysregulation, including altered glucose metabolism and impaired ketone body utilization, is a hallmark of gliomas. Recent preclinical and clinical investigations have explored the ketogenic diet (KD) as a potential therapeutic avenue. Animal studies suggest a survival benefit with KD, albeit clinical evidence remains inconclusive due to heterogeneous study designs and challenges in diet adherence. Metabolic imaging studies provide insight into KD-induced metabolic shifts within tumors and normal brain tissue. However, interpretation of data is complex, warranting further investigation. Challenges associated with sustaining KD adherence, especially in GBM patients, underscore the need for comprehensive support strategies. Mitochondrial enzyme expressions, specifically BDH1 and OXT1, may influence glioma cell dependency on glucose and ketone bodies, offering potential as therapeutic targets and prognostic markers. Future research integrating patient selection based on metabolic profiles holds promise for personalized treatment approaches. Overall, while the KD shows potential in GBM management, its clinical efficacy requires further elucidation through rigorous studies and multidisciplinary support frameworks.
Introduction
Glioblastoma (GBM) stands out as the most aggressive and most frequent primary malignant brain tumor, characterized by resistance to chemo, radiotherapy, and immunotherapy. The established standard of care involves extensive tumor resection, followed by chemo-radiotherapy protocol (Stupp or Perry regimen for elderly), or exclusive radiation therapy or chemotherapy only in selected patients according to age, performance status, and methylation status of MGMT promoter; in patients in poor conditions, best supportive therapy may be indicated. Despite therapy adherence, patients experience a median survival ranging from 14 to 21 months, with a 5-year survival rate of approximately 10% [1]. Despite extensive research in the last 20 years, this dismal prognosis underscores the need for innovative/additional treatment modalities.
Alterations in metabolism are a distinctive feature in glioma as well as in other malignant tumors. Since the 20ies the work by Warburg has underscored the preferential use by cancer cells of extra-mitochondrial glycolysis even in the presence of oxygen, leading to excess lactate production and non-efficient energy production via the Krebs cycle. In this conceptual framework, cancer cells would be glucose-dependent and possibly also not fully able to shift to other metabolic sources – i.e. ketone bodies – for energy production. On the other hand, normal cells, and especially normal brain cells, are indeed able to utilize ketone bodies to fulfill their energetic needs. Such an ability is supposed to have an evolutionary meaning, leading to the possibility of resisting periods of starvation without a significant metabolic derangement in neuronal function. For these reasons, there is an ongoing research effort aimed at targeting gliomas via metabolic approaches.
The evidence for damaged/inefficient metabolism via the TCA cycle in gliomas and of their supposed inability to metabolize ketone bodies is provided by electron microscopy studies showing morphological alterations in the structure of mitochondria in GBM patients [2,3], as well as by immunohistochemistry [4] and PCR studies showing reduced expression/concentration of ketolytic enzymes in the context of these tumors [5].
Metabolic defects may be interrelated with genetic abnormalities in gliomas; in this context, a particular case is that of IDH1 mutations. These mutations have been shown to be a hallmark in astrocytomas and oligodendrogliomas, being detected in gliomas in younger patients with a better life expectancy and response to treatment as compared with IDH1 wild-life cases with the same histological grading. Mutations lead to an intracellular accumulation of 2-hydroxyglutarate which acts as an oncometabolite in these tumors [6] and leads to an increased influx of glutamine-derived glutamate in the mitochondria in order to balance the reduced levels of alpha-ketoglutarate [7]. Glutamine, an abundant amino acid, plays a crucial role in cellular health and function. It serves as a mitochondrial energy source, a substrate for nonessential amino acid synthesis, a nitrogen source for nucleotide biosynthesis, and contributes to lipid synthesis and glutathione production [8].
A recent trial with a molecule targeting IDH1/IDH2 mutations (vorasidenib) has shown promising results in these tumors, delaying disease progression in grade 2, IDH-mutated glioma [9]. However, IDH1-2 mutated gliomas represent a low percentage of the total amount of these cancers, leaving treatment in the most aggressive ones, ie glioblastoma, still largely unsatisfactory despite tremendous efforts in research involving chemotherapy, radiation treatments, targeted therapies, immunotherapy [10]. Based on this premise, the deprivation of tumor cells through reduced glucose availability emerges as an additional therapeutic avenue. Indeed, the escalating volume of research papers indexed on PubMed over the past decade, utilizing keywords “cancer” and “ketogenic diet” (KD), attests to the mounting interest among clinicians and researchers in this field (Figure 1).
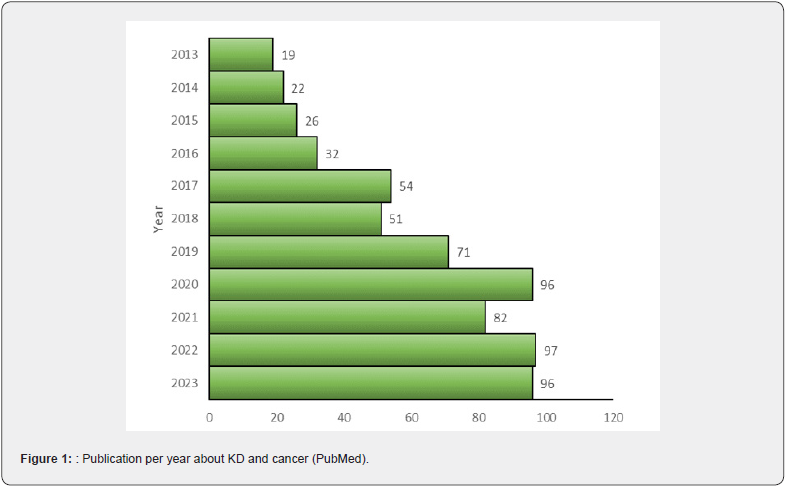
The KD is a high-fat, low-carbohydrate diet that aims to shift the body’s metabolism from glucose to ketones for energy. The metabolic shift can be obtained using various KD schemes in which the percentage of fat ranges from 50% to 90%. As a -matter of fact, the Classic Ketogenic Diet (CKD) consists of a 3:1 or 4:1 ratio of fat to protein and carbohydrate sources, while in the Modified Atkins Diet (MAD) approximates a 1:1 to 2:1 macronutrient ratio. Alternatively, a medium-chain-triglycerides (MCT) diet has been proposed in which at least 30% of triglycerides are medium –chain-triglycerides [11]. A primary concern in this context revolves around the safety of the proposed diets and the challenges associated with diet adherence. While adherence to KD in animal studies poses no significant hurdles, most clinical studies conducted thus far in patients have focused on dietary regimens of relatively brief durations, typically spanning 2-3 months. Even with such a short duration, these investigations have revealed suboptimal adherence to the prescribed schedules.
KD and glioblastoma in pre-clinical and clinical studies
Animal studies
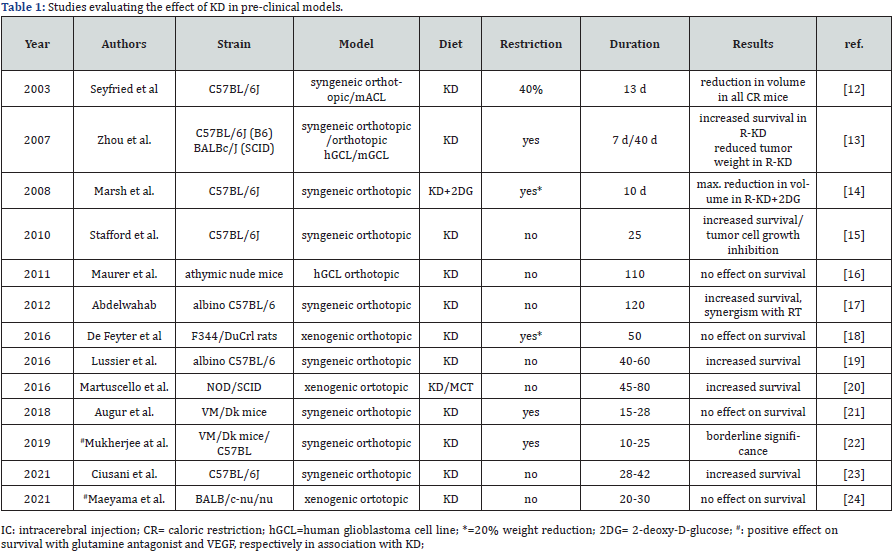
Table 1 reports the main pre-clinical studies conducted with dietary approaches in glioma models [12-24]; studies up to 2018 were previously reviewed by Weber [25]. These studies in cell cultures and in experimental glioma models have shown some effect of ketogenic diet – either alone or with caloric restriction – in prolonging survival in animal [13,15,19,20,23] and possibly potentiate the effects of radiotherapy models [17]. Out of the thirteen studies listed in table 1, nine (69.2%) reported an increased survival effect of KD while four did not. Of note, all the nine studies in which an effect of KD was detected, used animal models based on C57BL/6N-GL261 mice.
Discrepant results may be due to the highly variable experimental models; use of human glioblastoma cells in nude mouse [26,27], use of human IDH1 wt or IDH1 mutated cell lines in nude mice or rodent IDH1 wt/mut intracranially in C57BL/6J mice [28], murine glioblastoma cell lines in VM/Dk and in C57BL/6J mice [22], and murine glioblastoma cell line in C57BL/6N mice [23]. To our knowledge, GL261 cell line, which is routinely used in the C57B6 model, has not been analyzed as far as expression of ketolytic enzymes is concerned. Also, caloric restriction per se has been shown to prolong survival in a mouse glioma model [29]; however, it does not seem to play a pivotal role in animal studies since both in studies showing increased survival and in negative ones, calories restriction was equally represented (about 50%, Table 1).
Clinical studies
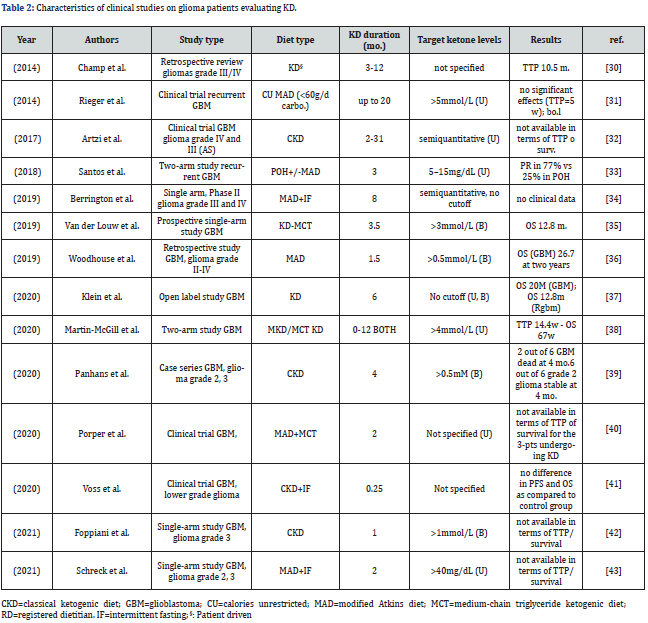
Clinical studies investigating KD approaches in adult patients with glioma are reported in Table 2 [30-43].
The 14 trials identified are heterogeneous: in fact, they do not only differ as for the type of diet, but also for the adoption or not of caloric restriction (sometimes in the form of intermittent fasting, as in the study by Schreck [43], or for the highly variable duration of the intervention; this latter point is of paramount relevance, spanning from 9 days as in the ERGO-2 trial to 20 months (1 patient) in the trial by Porper [40, 41]. Of note, only 3 of the 14 trials planned an intervention for longer than 4 months. Differences in the diet setting (KD variation, time since diagnosis), assessment of diet adherence (urine vs blood ketone levels, self-reported adherence and tolerance), home-made food versus nutraceutical industry products and limited trial duration or prolongation of the intervention in single patients, make it very hard to draw meaningful conclusions from the research effort.
Concerning tolerability, it has to be stressed that dropout rates are significant in patients undergoing KD in all its variants although with different rates (up to 60%) [44]. Most dropouts are related to heavy diet burden affecting the patient and/or the caregiver. Additional reasons include social challenges (difficulty adhering to the diet in social settings), side effects and psychological factors (lack of support, motivation, or difficulty adapting to the new dietary pattern).
As far as potential alteration in metabolic parameters, some studies have documented loss of body weight and slight hypoglycemia, whereas concerns for increased cholesterol and triglyceride levels in blood seem not to be supported by available evidence with the caveat of the duration of dietary intervention [44]. Brain metabolic imaging with MRS (MR-spectroscopy) provides an interesting tool to investigate shifts in the metabolic profile of brain tumors in vivo and potentially assess the effect of metabolic therapy approaches. Two studies have provided insight in this respect [32,34]. In the former study, 5 patients undergoing KD treatment (1 with gliomatosis with diet alone and 4 with recurrent glioblastoma in combination with bevacizumab) were followed with serial MR-spectroscopy, which was also targeted at areas of normal- appearing white matter; in the 2 patients adhering to the diet, acetone and acetoacetate were detected in 4 MR spectra (3 in the NAWM and 1 in the lesion, 4 and 25 months respectively after initiation of the diet).
In the study by Berrington et al., 3T MR spectroscopy was able to detect increase in acetone and beta-hydroxybutyrate in the lesion by week 8 of the diet, and an increase in acetone was also detected in the contralateral normal-appearing brain at this time point [34]. Although both studies do suggest that KD induces detectable changes in the metabolic profile both within the tumor area and in the normal-appearing brain, interpretation of the data is not straightforward, in as much as increases in the peak of a ketone body may reflect both an increased production by the tumor itself or an inability to metabolize the ketone body with subsequent increase in local concentration. Larger and more rigorous clinical and imaging studies with MRS are needed to establish the safety and efficacy of this approach and its potential mechanisms in vivo.
The challenges of KD
Maintaining proper nutrition is crucial during cancer treatment, and following a ketogenic diet can be challenging, especially for cancer patients who may already be dealing with appetite loss, nausea, and other treatment-related side effects. In fact, while animal studies demonstrate the feasibility of maintaining a KD for the entire experimental duration (30-80 days), clinical studies often involve relatively short periods of KD implementation. Insights from studies on epilepsy patients suggest that, in carefully selected cases, KD can be sustained for over 24 months with minimal or no side effects [45,46]. However, the potential for such an extended KD duration to impact the survival of patients with GBM remains uncertain. Further exploration is needed to ascertain whether a prolonged adherence to a KD might contribute to extending the survival rates of GBM patients.
For individuals dealing with GBM, maintaining adherence to a stringent diet, may pose a significant challenge also due to its lack of palatability. To enhance the understanding of the impact of the KD in GBM, future studies should adopt a comprehensive, multidisciplinary approach. This approach goes beyond ensuring standard care for the tumor but also addressing the various complications that these patients may encounter. However, given the poor survival of these patients, we must consider that the burden of complications associated with KD may not be paramount. Moreover, it should encompass a motivational component, facilitated through support groups led by a diverse team of professionals, including psychologists, nutritionists, physiotherapists, as well as culinary experts and social media communities. This wide approach aims to not only address the physical aspects of treatment but also provide emotional and practical support, making the dietary regimen more manageable for patients and their caregivers.
Conclusion
While studies in animal models propose a potential role for the KD in extending survival among GBM-implanted animals, this beneficial effect lacks substantial confirmation in clinical studies. The pivotal involvement of mitochondria in utilizing beta-hydroxybutyrate within the energetic metabolism of glioma cells suggests that distinct expressions of the BDH1 (encoding mitochondrial hydroxybutyrate dehydrogenase 1) and OXT1 (encoding succinyl-CoA:3-oxoacid CoA transferase 1) could play a crucial role in determining the glucose dependency of glioma cells. Lower BDH1 and OXT1 expression could limit BHB utilization and increase glucose dependence [47]. On the other hand, cells exhibiting elevated expression of these genes may demonstrate heightened proficiency in harnessing BHB, thereby potentially diminishing their dependence on glucose. Such a phenomenon could hold multifaceted implications, possibly serving as a basis for therapeutic interventions, patient classification, or prognostic indicators. This, in turn, might influence the sensitivity of the patient to treatment and aid in identifying specific groups of patients who are more likely to derive benefits from the KD. Indeed, the heterogeneity observed in gliomas extends to enzymatic activities within the mitochondria. Consequently, future studies could enhance their design by incorporating the selection of patients based on their tumor metabolic profile. This approach would contribute to a more extended understanding of the potential effectiveness of the KD in treating glioblastoma, allowing for targeted and personalized interventions.
Funding
This work was partially supported by the Italian Ministry of Health, Ricerca Corrente (2021-2023 AD, AP, AS, EC).
References
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, et al. (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5): 459-466.
- Arismendi-Morillo G, Castellano-Ramirez A, Seyfried TN (2017) Ultrastructural characterization of the Mitochondria-associated membranes abnormalities in human astrocytomas: Functional and therapeutics implications. Ultrastruct Pathol 41(3): 234-244.
- Deighton RF, Le Bihan T, Martin SF, Gerth AMJ, McCulloch M, et al. (2014) Interactions among mitochondrial proteins altered in glioblastoma. J Neurooncol 118(2): 247-256.
- Chang HT, Olson LK, Schwartz KA (2013) Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond) 10(1): 47.
- Sperry J, Condro MC, Guo L, Braas D, Vanderveer-Harris N, et al. (2020) Glioblastoma Utilizes Fatty Acids and Ketone Bodies for Growth Allowing Progression during Ketogenic Diet Therapy. iScience 23(9): 101453.
- Gonzalez N, Asad AS, Gomez Escalante J, Pena Agudelo JA, Nicola Candia AJ, et al. (2021) Potential of IDH mutations as immunotherapeutic targets in gliomas: a review and meta-analysis. Expert Opin Ther Targets 25(12): 1045-1060.
- Waitkus MS, Diplas BH, Yan H (2015) Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol 18(1): 16-26.
- Lane J, Brown NI, Williams S, Plaisance EP, Fontaine KR (2021) Ketogenic Diet for Cancer: Critical Assessment and Research Recommendations. Nutrients 13(10): 3562.
- Mellinghoff IK, van den Bent MJ, Blumenthal DT, Touat M, Peters KB, et al. (2023) Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med 389(7): 589-601.
- Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, et al. (2020) Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol 6(7): 1003-1010.
- Schwartz KA, Noel M, Nikolai M, Chang HT (2018) Investigating the Ketogenic Diet As Treatment for Primary Aggressive Brain Cancer: Challenges and Lessons Learned. Front Nutr 5: 11.
- Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P (2003) Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer 89(7): 1375-1382.
- Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, et al. (2007) The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 4: 5.
- Marsh J, Mukherjee P, Seyfried TN (2008) Drug/diet synergy for managing malignant astrocytoma in mice: 2-deoxy-D-glucose and the restricted ketogenic diet. Nutr Metab (Lond) 5: 33.
- Stafford P, Abdelwahab MG, Kim DY, Preul MC, Rho JM, et al. (2010) The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond) 7: 74.
- Maurer GD, Brucker DP, Bahr O, Harter PN, Hattingen E, et al. (2011) Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 11: 315.
- Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, et al. (2012) The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One 7(5): e36197.
- De Feyter HM, Behar KL, Rao JU, Madden-Hennessey K, Ip KL, et al. (2016) A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth. Neuro Oncol 18(8): 1079-1087.
- Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, et al. (2016) Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 16: 310.
- Martuscello RT, Vedam-Mai V, McCarthy DJ, Schmoll ME, Jundi MA, et al. (2016) A Supplemented High-Fat Low-Carbohydrate Diet for the Treatment of Glioblastoma. Clin Cancer Res 22(10): 2482-2495.
- Augur ZM, Doyle CM, Li M, Mukherjee P, Seyfried TN (2018) Nontoxic Targeting of Energy Metabolism in Preclinical VM-M3 Experimental Glioblastoma. Front Nutr 5: 91.
- Mukherjee P, Augur ZM, Li M, Hill C, Greenwood B, et al. (2019) Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun Biol 2: 200.
- Ciusani E, Vasco C, Rizzo A, Girgenti V, Padelli F, et al. (2021) MR-Spectroscopy and Survival in Mice with High Grade Glioma Undergoing Unrestricted Ketogenic Diet. Nutr Cancer 73(11-12): 2315-2322.
- Maeyama M, Tanaka K, Nishihara M, Irino Y, Shinohara M, et al. (2021) Metabolic changes and anti-tumor effects of a ketogenic diet combined with anti-angiogenic therapy in a glioblastoma mouse model. Sci Rep 11(1): 79.
- Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, et al. (2020) Ketogenic diet in the treatment of cancer - Where do we stand? Mol Metab 33: 102-121.
- Ji CC, Hu YY, Cheng G, Liang L, Gao B, et al. (2019) A ketogenic diet attenuates proliferation and stemness of glioma stem‑like cells by altering metabolism resulting in increased ROS production. Int J Oncol 56(2): 606-617.
- Sperry J, Condro MC, Guo L, Braas D, Vanderveer-Harris N, et al. (2020) Glioblastoma Utilizes Fatty Acids and Ketone Bodies for Growth Allowing Progression during Ketogenic Diet Therapy. iScience 23(9): 101453.
- Javier R, Wang W, Drumm M, McCortney K, Sarkaria JN, et al. (2022) The efficacy of an unrestricted cycling ketogenic diet in preclinical models of IDH wild-type and IDH mutant glioma. PLoS One 17(2): e0257725.
- Jiang YS, Wang FR (2013) Caloric restriction reduces edema and prolongs survival in a mouse glioma model. J Neurooncol 114(1): 25-32.
- Champ CE, Palmer JD, Volek JS, Werner-Wasik M, Andrews DW, et al. (2014) Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol 117(1): 125-131.
- Rieger J, Bahr O, Maurer GD, Hattingen E, Franz K, et al. (2014) ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol 44(6): 1843-1852.
- Artzi M, Liberman G, Vaisman N, Bokstein F, Vitinshtein F, et al. (2017) Changes in cerebral metabolism during ketogenic diet in patients with primary brain tumors: 1H-MRS study. J Neurooncol 132(2): 267-275.
- Santos JG, Da Cruz WMS, Schonthal AH, Salazar MD, Fontes CAP, et al. (2018) Efficacy of a ketogenic diet with concomitant intranasal perillyl alcohol as a novel strategy for the therapy of recurrent glioblastoma. Oncol Lett 15(1): 1263-1270.
- Berrington A, Schreck KC, Barron BJ, Blair L, Lin DDM, et al. (2019) Cerebral Ketones Detected by 3T MR Spectroscopy in Patients with High-Grade Glioma on an Atkins-Based Diet. AJNR Am J Neuroradiol 40(11): 1908-1915.
- van der Louw EJTM, Reddingius RE, Olieman JF, Neuteboom RF, Catsman-Berrevoets CE (2019) Ketogenic diet treatment in recurrent diffuse intrinsic pontine glioma in children: A safety and feasibility study. Pediatr Blood Cancer 66(3): e27561.
- Woodhouse C, Ward T, Gaskill-Shipley M, Chaudhary R (2019) Feasibility of a modified Atkins diet in glioma patients during radiation and its effect on radiation sensitization. Curr Oncol 26(4): e433-e438.
- Klein P, Tyrlikova I, Zuccoli G, Tyrlik A, Maroon JC (2020) Treatment of glioblastoma multiforme with "classic" 4:1 ketogenic diet total meal replacement. Cancer Metab 8(1): 24.
- Martin-McGill KJ, Marson AG, Tudur Smith C, Young B, Mills SJ, Cherry MG, et al. (2020) Ketogenic diets as an adjuvant therapy for glioblastoma (KEATING): a randomized, mixed methods, feasibility study. J Neurooncol 147(1): 213-227.
- Panhans CM, Gresham G, Amaral LJ, Hu J (2020) Exploring the Feasibility and Effects of a Ketogenic Diet in Patients With CNS Malignancies: A Retrospective Case Series. Front Neurosci 14: 390.
- Porper K, Shpatz Y, Plotkin L, Pechthold RG, Talianski A, et al. (2021) A Phase I clinical trial of dose-escalated metabolic therapy combined with concomitant radiation therapy in high-grade glioma. J Neurooncol 153(3): 487-496.
- Voss M, Wenger KJ, von Mettenheim N, Bojunga J, Vetter M, et al. (2022) Short-term fasting in glioma patients: analysis of diet diaries and metabolic parameters of the ERGO2 trial. Eur J Nutr 61(1): 477-487.
- Foppiani A, De Amicis R, Lessa C, Leone A, Ravella S, et al. (2021) Isocaloric Ketogenic Diet in Adults with High-Grade Gliomas: A Prospective Metabolic Study. Nutr Cancer 73(6): 1004-1014.
- Schreck KC, Hsu FC, Berrington A, Henry-Barron B, Vizthum D, et al. (2021) Feasibility and Biological Activity of a Ketogenic/Intermittent-Fasting Diet in Patients With Glioma. Neurology 97(9): e953-e963.
- Guo A, Asztely F, Smits A, Jakola AS (2023) Methodological Approaches to Ketogenic Dietary Treatments in Glioma Patients from a Nutritional Point of View. Nutr Cancer 75(1): 112-122.
- El-Rashidy OF, Nassar MF, Shokair WA, El Gendy YGA (2023) Ketogenic diet for epilepsy control and enhancement in adaptive behavior. Sci Rep 13(1): 2102.
- Chang P, Augustin K, Boddum K, Williams S, Sun M, et al. (2016) Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 139(Pt 2): 431-433.
- Guntuku L, Naidu VG, Yerra VG (2016) Mitochondrial Dysfunction in Gliomas: Pharmacotherapeutic Potential of Natural Compounds. Curr Neuropharmacol 14(6): 567-583.






























