- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Conjunctival Melanoma: A Clinico-Pathologic Review
Sally Owens1,2,3*, Emily Greenan4*, Noel Horgan4,5 and Susan Kennedy1,2,3
1National Ophthalmic Pathology Laboratory, Royal Victoria Eye and Ear Hospital, Dublin, Ireland
2Research Foundation, Royal Victoria Eye and Ear Hospital, Dublin, Ireland
3School of Biotechnology, Dublin City University, Dublin, Ireland
4Department of Ophthalmology, Royal Victoria Eye and Ear Hospital, Dublin, Ireland
5Department of Ophthalmology, St Vincent’s University Hospital, Dublin, Ireland
Submission: February 10, 2024; Published: February 20, 2024
*Corresponding Address: Dr Emily Greenan, Department of Ophthalmology, Royal Victoria Eye and Ear Hospital, Dublin, Ireland, Email: emily.greenan@rveeh.ie
How to cite this article: Sally O, Emily G, Noel H, Susan K. Conjunctival Melanoma: A Clinico-Pathologic Review. Canc Therapy & Oncol Int J. 2024; 26(1): 556179. DOI:10.19080/CTOIJ.2024.26.556179
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Abstract
Conjunctival melanoma is a rare but life-threatening malignancy that arises from melanocytes in the basal layer of the conjunctival membrane. It only accounts for 2% of ocular melanomas. Its genetic makeup is distinct from uveal melanoma. It has more similarities to cutaneous melanoma in both patterns of spread and genetics. It predominantly affects Caucasians and the elderly. It arises most commonly from primary acquired melanosis but can also arise from a pre-existing conjunctival naevus or de novo. This review aims to outline the predisposing conditions, epidemiology, survival, histology, genetics, and current therapy for practising pathologists who may have limited experience with this tumour type.
Keywords: Conjunctival melanoma; Primary acquired melanosis; Histopathologic features, Histopathological staging; Immunotherapy; Molecular targeted therapies
Keywords: AJCC: American Joint Committee on Cancer; ATRX: Alpha-Thalassemia/Mental Retardation, X-linked; BRAF: B-Raf Proto-Oncogene, Serine/Threonine Kinase; c-KIT: KIT Proto-Oncogene, receptor tyrosine kinase; C-MIL: Conjunctival Melanocytic Intraepithelial Lesion; CM: Conjunctival Melanoma; CMIN: Conjunctival Melanocytic Intraepithelial Neoplasia; GNA11: Guanosine Nucleotide-Binding Protein Alpha-11; GNAQ: Guanosine Nucleotide-Binding Protein G; MAPK: Mitogen-Activated Protein Kinase; MEK: Mitogen-Activated Extracellular Signal Related Kinase; MLPA: Multiple Ligation-Dependent Probe Amplification; NF1: Neurofibromin 1; PAM: Primary Acquired Melanosis; SEER: Surveillance, Epidemiology and End Results; TERT: Telomerase Reverse Transcriptase; TNM: Tumour, Node, Metastasis; UV: Ultraviolet
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Introduction
Conjunctival melanocytic lesions including conjunctival melanoma (CM) are rare. Most general pathologists will be unfamiliar with the clinical terminology and histopathologic features of this neoplasm as it accounts for a very small percentage of all surfaces exposed melanoma and for a very small percentage of peri-ocular melanoma. CM accounts for 2% of all eye tumours and 5% of melanomas arising in the periocular region [1]. Most of the latter are uveal melanomas originating inside the eyeball and have a completely different genetic basis, metastatic pathway, and survival to CM. CM will first spread to the regional lymph nodes rather than to the liver which is typical of uveal melanoma [2]. Molecular investigations show that CM shares more traits with skin or other mucosal melanomas rather than uveal melanoma [3]. It is a hybrid of both skin and mucosal melanomas [3]. CM occurs most commonly on the eyeball, but malignant tumours may arise in the palpebral (inner lining of eyelid) and forniceal (junction of palpebral eyelid and eyeball) conjunctiva and the caruncle (specialized skin at most medial aspect of eyelids) [2]. CM has a 10 year mortality rate of approximately 30%. Metastatic disease may respond to check point inhibitors or targeted molecular therapies which have proven successful in halting progression of cutaneous melanoma.
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Epidemiology
CM is typically more common in Caucasians than other races. A US study undertaken by Hu (2008) [4] us-ing an US surveillance, epidemiology, and end results (SEER) database found that the rate for black indi-viduals was three times lower than for whites. The overall crude incidence rate was 0.53 per 1-million-person years in that study. According to another SEER database analysis, the incidence of cases increased by 101% over a 27-year period, between 1973 and 1999 and an increase of 295% in white males older than 60 [5]. Isager (2005) [6] summarised 14 incidence studies, mostly single country/region studies, which varied from 0.1 to 0.9 per 1-million-person years encompassing different periods and geographic sites. The age-adjusted incidence of CM rose by sevenfold from 1960 to 2005 in Sweden [7] and increased twofold between 1967 and 2000 in Finland [8]. This reported incidence rise over the past few decades, in parallel with the rise of cutaneous melanoma, possibly indicates an association between CM and ultravio-let (UV) exposure [7,8]. Studies on survival from Sweden and Denmark show a relatively stable 5- and 10-year survival [9]. Isiger [10] showed 5- and 10-year survival of 88% and 76% respectively. Kujala [11] reported a 10-year melanoma related survival of 61%.
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Presentation
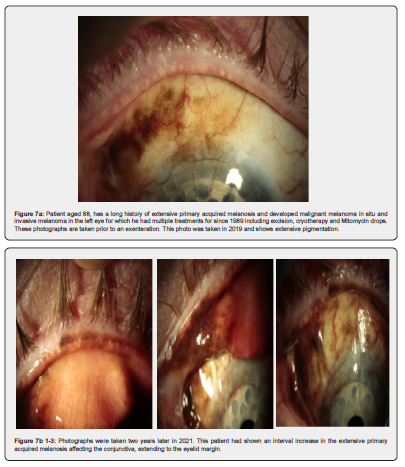
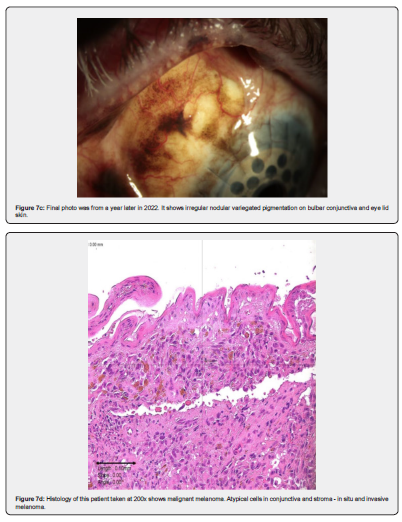
CM most commonly presents as a variably pigmented vascularised, macule, plaque, or mass at any site of the conjunctiva. The risk factors for CM are not entirely established [12] but there are two well estab-lished pre-existing conditions, primary acquired melanosis (PAM), and naevi. Approximately 7% arise in association with a pre-existing naevus, 19% arise de novo, and 74% of CM are preceded by PAM [13]. Ex-amples of these can be seen in Figures 1 & 2, and Figures 4-7 respectively. The largest detailed follow up studies of PAM [14] and conjunctival naevi [15] are both from the Shields group in Philadelphia. PAM accounted for 21% of melanocytic lesions in their ocular oncology service. They believe that the occur-rence in the general population may be higher than generally appreciated because many patches are small and do not come to clinical attention. Evidence for this hypothesis was provided by a prospective study of all patients over the age of 10 attending a corneal clinic for unrelated conditions by Gloor and Alexander [16]. They found PAM in 36% of adult Caucasians.
In a detailed retrospective series of 113 patients with at least 3 years of follow up [14], Shields group characterised the lesions and identified factors associated with progression. PAM was de-fined clinically “as one or more patches of acquired asymmetric flat, discrete non cystic conjunctival or corneal brown pigmentation of at least 1mm in diameter that lacks the typical features of localized naevus or racial melanosis” [14].
The mean patient age was 56 years (15-90 years). 62% were female, 96% Caucasian but of note only 4% of the population of patients in the study were non-Caucasian. A bulbar or eyeball location was seen in 91%, limbal (corneal – conjunctival interface) in 55% and cornea 23%, whereas forniceal (13%), palpebral (12%) and caruncle (11%) were less common. The mean size was 3 clock hours. 194 (62%) were initially monitored by observation based on size (median 4mm). 107 (34%) were treated by incisional or excisional biopsy and cryotherapy. Of the patients treated by observation, PAM enlargement occurred in 16% and progression to melanoma in 5% within a mean of 56 months. Of those managed by biopsy and cryotherapy, PAM recurrence was detected in 27% and progression to melanoma in 3% with a mean interval of 39 months. Histologic evaluation for those treated by biopsy, showed that when a diagnosis of PAM with no atypia was made, there was a recurrence rate of 11%. A 26% rate for PAM with mild atypia, and 50% recurrence for PAM with severe atypia. Significantly, there were no cases of progression to melanoma in patients with no or mild atypia. Melanoma occurred in 13% of cases of PAM with severe atypia.
The extent of PAM in clock hours was shown to be the most important factor in progression to melanoma at 15 years assessed by Kaplan–Meier estimates (21%) in the group monitored by observation only. The group treated by biopsy and cryotherapy had a 15-year estimated progression rate of 11% suggesting that excision may be curative in many cases. The Shields study [14] documents the slow but potentially malignant evolution of PAM to melanoma and highlights the importance of the diameter of the lesion in the initial assessment of risk.
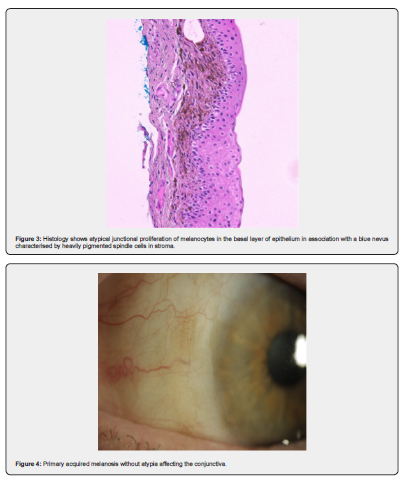
The Shields group (2004) also followed a large group of conjunctival naevi patients over a 28-year time span [15]. They had 410 patients with conjunctival naevi referred to their practice. Only 3 patients devel-oped malignant melanoma from a pre-existing compound naevus in two cases and a blue naevus in one case. Figure 3 shows an example of blue naevus. They commented that this rate of transformation (0.7%) probably overstated the number of cases as they are a tertiary referral centre. Nooregaard [17] found that melanomas originated from a naevus in 16%, PAM in 36% and de novo in 48%. These figures from Denmark suggest a higher rate of conversion than the Shields study [14].
Teaching Point: PAM with no or with mild atypia is unlikely to progress to melanoma however PAM with severe atypia is at high risk of progression to melanoma. The risk increases when the size is >4mm.
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Clinical diagnosis of melanoma
Any adult patient with a pigmented lesion on the ocular surface can be suspected of having either CM or naevus. CM patients present, on average, in the 5th decade with an age range of 11-89 years although it is very rare in childhood [9,18].
Risk factors for progression summarised in a 2021 study include older age, history of prior conjunctival surgery and an advanced American Joint Committee on Cancer (AJCC) category [19]. The differential diag-nosis of any adult patient includes a benign naevus, PAM, metastatic melanoma of other sites, other be-nign and malignant tumours containing pigment. Once clinically assessed and considered suspicious, the patient has a slit lamp examination, examining the whole conjunctival surface, with eversion of the upper eyelid and tarsus. They will also undergo a dilated fundus examination. A complete physical examination with the preauricular, postauricular, parotid, sub-mandibular and cervical lymph nodes palpation is performed. Slit lamp photography is used to record any modifications to the conjunctival surface. Anterior segment optical coherence tomography enhances diag-nostic accuracy and can assist in planning surgery [12,20,21].
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Technical management of conjunctival histopathology samples
The histologic sample should be placed on a paper mount, to avoid disruption of its morphology, such as the occurrence of scrolling, which may interfere with the diagnosis. If oriented the sample can be “differentially inked” and “bread loafed” and submitted in its entirety and individual margins can be assessed.
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Histology
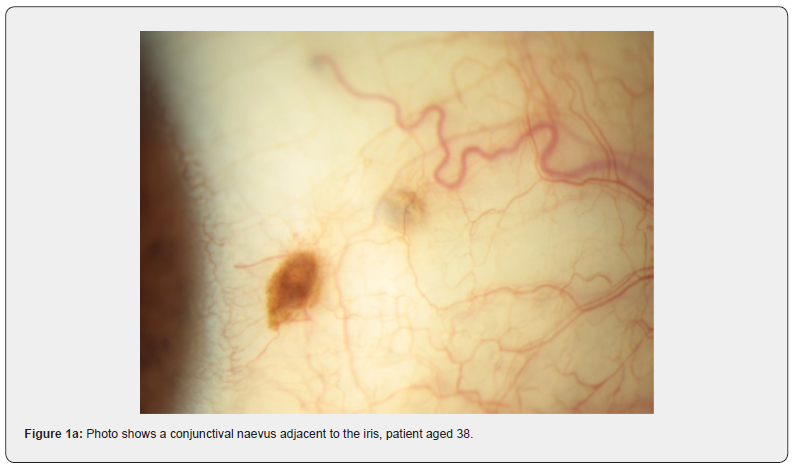
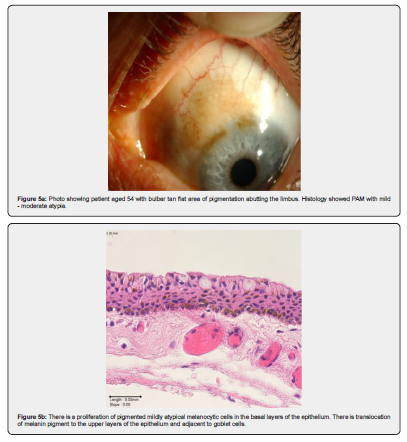
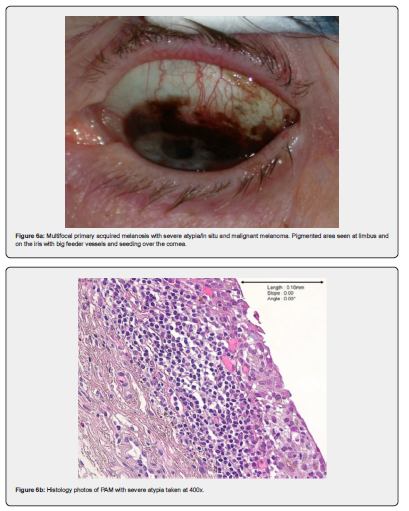
Naevi are benign melanocytic proliferations which on histology can be junctional, stromal, or combined. See Figure 1a & 1b as an example of a junctional naevus. The melanocytes are small, with little cytologic atypia and are associated with cystic inclusions of epithelium. Maturation is seen in stromal lesions [22]. PAM with mild atypia, example in Figure 5a and 5b, shows melanocytic hyperplasia with increased nu-clear size and irregularity of cells. There may be dispersed pigment in more superficial layers of the epithelium [22]. Increasing degrees of PAM show melanocytic cells throughout the full thickness of the epithelium [22]. Figures 6a-b and Figures 7a-d show PAM with severe atypia and malignant melanoma.
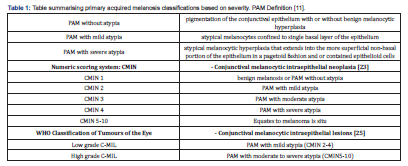
The CMIN (conjunctival melanocytic intraepithelial neoplasia) is a scoring system that evaluates melano-cytic growth pattern, degree of cellular atypia and mitotic figures. The extent of horizontal and vertical epithelial melanocytic spread is assessed. The advantages of this scoring system are that there is a repro-ducible basis for the diagnosis of PAM with severe atypia/melanoma in situ. However, in the case of very thick epithelium, which is often the case, vertical growth patterns cannot be considered [23]. The term in-traepithelial melanocyte proliferation with atypia has also been proposed instead of PAM [24]. In WHO Classification of Tumours of the Eye (2018) [25] it is proposed to grade melanosis as low/high grade C-MIL (conjunctival melanocytic intraepithelial lesions). The scoring system lacks the simplicity for pathologists and widespread recognition by clinicians of the more traditional PAM or CMIN terminology clinically [26]. The classifications for PAM are summarised in Table 1 below.
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Melanoma in situ
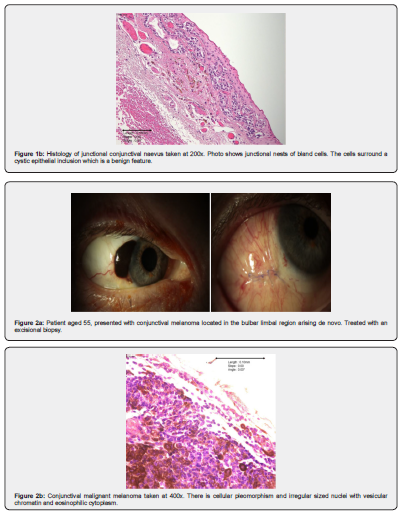
CM in situ is characterised by an intraepithelial proliferation of atypical melanocytes throughout all layers of the conjunctival epithelium (in situ component), often with confluent and irregular junctional nests [27]. When invasion through the basement membrane occurs, this is invasive malignant melanoma. There are four cell types seen in invasive melanoma: small polyhedral cells, large round epithelioid cells, balloon cells, and spindle cells [28-30].
An association has been found between epithelioid cell type and an increased morbidity [23]. The number of atypical mitotic figures is variable: >1/mm2 is a poor prognostic factor. Diagnostic features which are helpful include pagetoid spread of atypical cells in the epithelium, confluent nests, atypical melanocytes, radial and vertical extension of the process, inflammation adjacent to the basal layer of the lesion, and in-filtration into the sclera or cornea [12,30]. The finding of intralesional cystic invaginations of epithelium is a reassuring feature as this is only seen in benign nevi.
Immunohistochemical assays detecting Melan-A, S-100, and HMB-45 may be helpful in identifying infil-trating cells in the sometimes-heavy band like inflammatory infiltrate associated with CM [29,31]. Bcl-2 and HSP90 over expression, sox10 and PRAME overexpression [32], Ki67 score, and loss of p16 expression are all useful adjuncts [33-35]. None of them are prognostic. In contrast to cutaneous melanoma where proliferation loss of p16 or expression of the stem cell marker BMI have been shown to be prognostic, there are no similar studies in CM [36,37].
Histopathological staging using the tumour, node, metastasis (TNM) staging system of 8th edition of the AJCC staging manual [26] is based both on the thickness of the tumour and on location. Bulbar melanoma is categorised as pT1 if <0.2 mm in thickness and pT2 if it’s over 0.2 mm in thickness. It is pT3 if there is local invasion of globe, eyelid or orbit, nasolacrimal duct, or sinuses. By contrast non bulbar are pT2 a or b depending on thickness of less than 0.2 mm or greater than 0.2mm in thickness [29,38]. Mapping biopsies may be performed because CM is multifocal in 30% of cases and melanoma in situ in discontinuity with the main tumour has a higher rate of recurrence, possibly because it represents a field change [39]. Other adverse pathologic prognostic factors include ulceration, mitoses >1/mm2, lymphatic invasion and a heavy associated inflammatory infiltrate. Location is important. Whilst >90% are located on lateral bulbar conjunctiva, those located in the fornix, caruncle or eye lid margin have a worse prognosis.
Teaching Point: Cystic inclusions of the epithelium are a benign feature which are seen in nevi. Examination of the peri lesional melanoma may show PAM.
CM spreads locally on the ocular surface including on the cornea. It can also spread directly into the globe or orbit, nasolacrimal system, and sinuses [40,41]. It can metastasise through lymphatic and hematogenous spread. It usually spreads first through lymphatic drainage [42]. Nasal conjunctival tumours spread to the submandibular lymph nodes [43]. Tumours located elsewhere on the conjunctiva generally spread to the pre-auricular and deep cervical lymph nodes [43]. Sentinel lymph node excision is not performed as a routine [44].
Anastasios [39], Heindl [45], and Aziz [46] have proposed a clinical management algorithm suggesting consideration of sentinel lymph node biopsy based on if two or more of four high risk factors: non – limbal location, >2mm thickness, ulceration and >1 mitosis per mm2 on histology [46]. In a 2019 meta-analysis of sentinel lymph node in the management of CM, the authors concluded that although there could be potential benefit, at present there is not enough evidence to support sentinel lymph node biopsy as a mandatory part of CM management [47]. Most tumours are excised and there is no tumour bed for assessment. In analogy to skin melanoma CM may show angiotropic dissemination which appears responsible for microscopic satellite and in transit metastases. Angiotropic dissemination means extravascular migration of melanoma cells along vascular channels [48]. The common sites of distant me-tastasis are the lungs, the brain, the liver, the skin, and the gastrointestinal tract [41,49].
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Surgical management
For resectable CM, total surgical excision is preferred with a “tumour-free” margin of around 2-4 mm, along with supplemental cryotherapy to the lesional margins and if there is corneal involvement, alcohol corneal epitheliectomy is used [12,40,49]. These additional treatments are done in order to eliminate any tumour cells which have not been detected clinically and that could reside on the edge resection [49]. Cryotherapy is applied on the margins of the resection. The conjunctiva is raised to prevent scleral damage [12,21,50]. Alcohol epitheliectomy allows for eradication of corneal epithelial tumour that may be involved [49]. The Bowman layer of the cornea (found just beneath corneal epithelial basement membrane) must stay intact given its role as a natural barrier against deep tumour invasion. A dry surgical field is used to prevent tumour cells spreading [12].
Effective treatment of conjunctival melanomas is complicated by a high rate of local recurrence. In addition to surgical excision, adjuvant therapy such as brachytherapy, cryotherapy and topical chemotherapeutic or immunotherapeutic agents like Mitomycin C or interferon alpha-2-beta may be used for local control [12, 20,50]. When total surgical removal cannot remove all macroscopic tumours, incisional biopsy is cautiously advised [51]. When performing an incisional biopsy, the high risk of tumour spread should be considered. A “no touch” technique is the use of new instruments at each step of the procedure, it is advised to avoid tumour seeding [12,38,40,51].
It is the belief of some surgeons that the excision of the lamellar sclera causes an increased chance of melanoma recurrence with a chance of intraocular infiltration and leave the patient with scarring, and thus should be avoided [50]. The excision is closed by applying conjunctival rotational flaps, mucous membrane graft from the contralateral eye, the buccal membrane or by amniotic membrane transplantation. The benefits of an amniotic membrane allograft are its high immunological tolerance [52], and its ability to cover exposed sclera [31]. Established CM cases should be referred to an oncologist and monitored every year to detect any possible metastasis. It has been stated by Grimes et al, (2020) that the “Guidelines for the treatment of local conjunctival melanoma are well-established, but there are no standard efficacious therapies for metastatic disease” [53]. Assessment should include an ophthalmological examination and a physical examination paying special attention to the head and neck lymph nodes. It is also advisable to carry out an annual chest radiography, liver ultrasound scans, liver function tests, brain MRI and ab-dominal/chest CT scan. Due to likelihood of acquiring cutaneous melanoma and dysplastic nevi, it is ad-visable to carry out routine dermatological examinations [38].
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Prognosis - Genetics and molecular therapy
CM and uveal melanomas have a different molecular profile. In contrast to uveal melanoma, CM frequently have a high tumour mutational burden. CM mostly occurring on actinic damaged conjunctiva is analogous to lentigo malignant melanoma demonstrates an ultraviolet light-related damage signature representing a high number of somatic mutations due to cytosine to thymidine transitions which is not observed in mucosal or uveal melanoma [54]. In addition, GNAQ and GNA11 mutations, most frequently found in uveal melanoma are very rarely observed in CM [6]. In contrast, CM are similar to cutaneous melanoma and frequently show mutations in MAPK/ERK pathway and the PI3K/AKT pathways [53]. Most frequent driver mutations are NF1, BRAF or NRAS mutations [3,9]. Rossi et al. [55] demonstrated 50% of tumours have BRAF, 30% have NF1, and 20% have NRAS mutations. Griewank et al. (2013) found that of 78 pa-tients with CM BRAF V600E mutations occurred in 27% of tumour et al. (RAS mutations in 18% [3]. Multiplex ligation-dependant probe amplification (MLPA) has allowed for the BRAF V600E mutation to be detected in 50% of primary and metastatic conjunctival melanomas [3]. BRAF encodes a serine/threonine kinase in the mitogen-activated protein kinase (MAPK) pathway. Activating mutations in the BRAF gene result in continuous downstream activation of the MAPK cascade including mitogen-activated extracellu-lar signal related kinase (MEK), leading to uncontrolled cell proliferation [29].
NRAS is an oncogene in the Ras family. It encodes for the GTPase which once mutated, activates a signal transduction pathway that deregulates cell division [12]. As in cutaneous melanomas, the BRAF and NRAS mutations are mutually exclusive. NF1 mutations can occur in association with BRAF and NRAS. NF1 is a tumour suppressor gene that has a domain that deactivates Ras proteins. This in turn, decreases the downstream activation of MAPK in wild-type cells [56]. A mutated NF1 gene was found in 33% of conjunctival melanomas according to a recent study comprised of 63 tumours [57]. Mucosal melanomas carry NF1 mutations at an estimated rate of 8–18% [58,59]. 12–25% cutaneous melanoma tumours have NF1 mutations [60]. An ATRX mutation (25%) often co-occurs with an NF1 mutation [61].
In contrast to mucosal and acral cutaneous melanoma which often show a c-KIT mutation, c-KIT mutation has been found only in 2.2.-7% of CM. CM with c-KIT mutations have been found to have an association with older age of onset [62]. The c-KIT gene encodes CD117, which is a receptor tyrosine kinase that plays a role in the growth and survival of melanomas. Conjunctival melanomas share characteristic copy number alterations and chromosomal aberrations with cutaneous melanoma. Of 30 CM tumours, gains were found in 1q, 3p, 7, and 17q while losses were found in 9p, 10, 11, 12q, and 16q [3]. These alterations are common in cutaneous and mucosal melanoma [3].
Both CM and cutaneous melanoma may have TERT promotor mutations which have been associated with worse prognosis [63]. Different ethnic groups appear to have distinct pathways in tumorigenesis. This hypothesis has been supported by a study conducted on 53 Chinese conjunctival melanoma patients in which 11% had KIT mutations and the prevalence of the BRAF mutation was only at 8%, which is very low compared to other studies [63]. There are many other genetic alterations that been associated with con-junctival melanoma in small scale studies, but BRAF, NRAS and NF1 are the main genetic alterations to note when associated with targeted therapies.
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Molecular targeted therapies
Immunotherapy and molecular targeted therapies are nowadays the gold standard for unresectable or metastatic skin or mucosal melanoma [64]. The ability to target specific melanoma driver genes or immune modulating therapies, such as checkpoint inhibitors have revolutionised patient’s disease free and tumour specific survival. Excellent tumour targets are BRAF and MEK inhibitors in BRAF mutated melano-mas or drugs against c-KIT mutated melanomas [65].
Studies for skin melanoma have shown that melanomas with high tumour mutational burden respond excellently to immune checkpoint inhibitors such as PD-1, PD-L1 and/or CTLA-4. Ho-Seok Sa [66] summarised seven published conjunctival melanoma cases treated with systemic immune checkpoint inhibitors. In one of the studies by Sagiv et al. [67] five patients were treated with a PD-1 inhibitors, 4 with nivolumab and 1 with pembrolizumab. Four patients treated with nivolumab had no evidence of disease after completing treatment while the remaining one (treated with pembrolizumab) progressed 11 months after therapy.
Teaching point: BRAF, NRAS and NF1 mutations will guide targeted therapy.
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Conclusion
In summary, conjunctival melanocytic lesions have a distinct natural history, with location and size important clinical prognostic factors. Pathologists are key in assisting in providing surgeon with accurate prognostic histologic information and guiding further therapy by judicious use of molecular testing when a diagnosis of melanoma has been established. Common conjunctival melanocytic lesions in adults include benign naevi, PAM with or without atypia/melanoma in situ and invasive malignant melanoma. When melanoma is diagnosed the site, diameter, thickness, assessment of cell type including the presence of epithelioid cells, ulceration, mitoses, lymphatic invasion, and margin status are all important. Histologic examination of melanocytic lesion is key to determining of prognostic risk, as naevi, PAM without atypia or mild atypia has an excellent prognosis and does not require further surgical treatment. If atypia is present, the diameter of the lesion and severity of atypia as well as location allow accurate prognostication. Assessment of BRAF, NRAS, c- KIT mutation status in the primary tumour is useful to guide therapy of recurrent or unresectable tumours and metastatic tumour.
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
Conflict of Interest
The authors have no conflicts of interest to declare.
- Review Article
- Abstract
- Introduction
- MEpidemiology
- Presentation
- Clinical diagnosis of melanoma
- Technical management of conjunctival histopathology samples
- Histology
- Melanoma in situ
- Surgical management
- Prognosis - Genetics and molecular therapy
- Molecular targeted therapies
- Conclusion
- Conflict of Interest
- Acknowledgement
- References
References
- Kastelan S, Gverovic Antunica A, Beketic Oreskovic L, Jasminka Salopek Rabatic, Boris Kasun, et al. (2018) Conjunctival Melanoma - Epidemiolog-ical Trends and Features. Pathology Oncology Research : POR 24(4): 787-796.
- Koc I, Kıratlı H (2020) Current Management of Conjunctival Melanoma Part 1: Clinical Features, Diagno-sis and Histopathology. Turk J Ophthalmol 50(5): 293–303.
- Griewank KG, Westekemper H, Murali R, Monika Mach, Bastian Schilling, et al. (2013) Conjunctival Melanomas Harbor BRAF and NRAS Mutations and Copy Number Changes Similar to Cutaneous and Mucosal Melanomas. Clinical Cancer Research 19(12): 3143–3152.
- Hu DN, Yu G, McCormick SA, Finger PT (2008) Population-Based Incidence of Conjunctival Melanoma in Various Races and Ethnic Groups and Comparison with Other Melanomas. American Journal of Ophthalmology 145(3): 418-423.e1.
- Yu GP, Hu DN, McCormick S, Finger PT (2003) Conjunctival melanoma: Is It Increasing in the United States? American Journal of Ophthalmology 135(6): 800–806.
- Isager P, Osterlind A, Engholm G, S Heegaard, J Lindegaard, et al. (2005) Uveal and Conjunctival Malignant Melanoma in Denmark, 1943–97: Incidence and Validation Study. Ophthalmic Epidemiol 12(4): 223–232.
- Triay E, Bergman L, Nilsson B, C All-Ericsson, S Seregard, et al. (2009) Time Trends in the Incidence of Conjunctival Melanoma in Sweden. British Journal of Ophthalmology 93(11): 1524–1548.
- Tuomaala S, Eskelin S, Tarkkanen A, Kivela T (2002) Population-based Assessment of Clinical Character-istics Predicting Outcome of Conjunctival Melanoma in whites. Investigative Ophthalmology & Visual Science 43(11): 3399–3408.
- Larsen AC, Dahmcke CM, Dahl C, Volkert D Siersma, Peter B Toft, et al. (2015) A Retrospective Review of Conjunctival Melanoma Presen-tation, Treatment, and Outcome and an Investigation of Features Associated With BRAF Mutations. JAMA Ophthalmology 133(11): 1295–1295.
- Isager P, Engholm G, Overgaard J, Storm H (2006) Uveal and Conjunctival Malignant Melanoma in Den-mark 1943–97: Observed and Relative Survival of Patients Followed through 2002. Ophthalmic Epidemiology 13(2): 85–96.
- Kujala E, Tuomaala S, Eskelin S, Kivela T (2009) Mortality after Uveal and Conjunctival melanoma: Which Tumour Is More deadly? Acta Ophthalmologica 87(2): 149–153.
- Wong JR, Nanji AA, Galor A, Karp CL (2014) Management of Conjunctival Malignant melanoma: a Review and Update. Expert Rev Ophthalmol 9(3): 185–204.
- Shields CL, Markowitz JS, Belinsky I, Hal Schwartzstein, Nina S George, et al. (2011) Conjunctival Melanoma outcomes based on tumor origin in 382 consecutive cases. Ophthalmology 118(2): 389-395.e2.
- Shields JA, Shields CL, Mashayekhi A, Brian P Marr, Raquel Benavides, et al. (2007) Primary Acquired Melanosis of the conjunctiva: Expe-rience with 311 eyes. Trans Am Ophthalmol Soc 105: 61-71.
- Shields CL, Fasiuddin A, Mashayekhi A, Shields J (2004) Conjunctival Nevi: Clinical Features and Natural Course in 410 Consecutive Patients. Archives of Ophthalmology 122(2): 167-175.
- Gloor PA, Alexandrakis G (1995) Clinical Characterization of Primary Acquired melanosis. Investigative Ophthalmology & Visual Science 36(8): 1721–1729.
- Norregaard JC, Gerner N, Jensen O, Prause JU (1996) Malignant Melanoma of the conjunctiva: Occur-rence and Survival following Surgery and Radiotherapy in a Danish Population. Graefes Arch Clin Exp Ophthalmol 234(9): 569–572.
- McDonnell JM, Carpenter J, Jacobs P, W L Wan, J E Gilmore (1989)Conjunctival Melanocytic Lesions in Children. Ophthal-mology 96(7): 986–993.
- Vaidya S, Dalvin LA, Yaghy A, Richard Pacheco, Jerry A Shields, et al. (2020) Conjunctival melanoma: Risk Factors for Recurrent or New Tumor in 540 Patients at a Single Ocular Oncology Center. Eur J Ophthalmol 31(5): 112067212097039.
- Lim L, Madigan M, Conway R (2013) Conjunctival melanoma: a Review of Conceptual and Treatment Ad-vances. Clinical Ophthalmology 9(3): 521-531.
- Vora GK, Demirci H, Marr B, Mruthyunjaya P (2017) Advances in the Management of Conjunctival Mela-noma. Surv Ophthalmol 62(1): 26–42.
- Eagle RC (2017) Eye Pathology: an Atlas and Text. Wolters Kluwer, Philadelphia, USA.
- Damato B, Coupland SE (2008) Conjunctival Melanoma and melanosis: a Reappraisal of terminology, Classification and Staging. Clin Exp Ophthalmol 36(8): 786–795.
- Jakobiec FA, Pooja Bhat, Kathryn A Colby (2010) Immunohistochemical Studies of Conjunctival Nevi and Melanomas. Arch Ophthalmol 128(2): 174-183.
- Grossniklaus HE (2018) WHO Classification of Tumours of the Eye. Lyon: International Agency For Re-search On Cancer.
- Amin MB, Edge SB (2017) AJCC Cancer Staging Manual. Springer, Switzerland.
- Bresler SC, Simon CJ, Shields CL, Jonathan B McHugh, Anna M Stagner et al. (2021) Conjunctival Melanocytic Lesions. Arch Pathol Lab Med 146(5): 632–646.
- Missotten GS, Keijser S, De Keizer RJW, De Wolff-Rouendaal D (2005) Conjunctival Melanoma in the Netherlands: a Nationwide Study. Invest Ophthalmol Vis Sci 46(1): 75.
- Jakobiec FA, Folberg R, Iwamoto T (1989) Clinicopathologic Characteristics of Premalignant and Malig-nant Melanocytic Lesions of the Conjunctiva. Ophthalmology 96(2): 147–166.
- Zembowicz A, Mandal RV, Choopong P (2010) Melanocytic Lesions of the Conjunctiva. Arch Pathol Lab Med 134(12): 1785–1792.
- Chen Z, Yan J, Yang H, Wu Z, Pang Y, et al (2003) Amniotic Membrane Transplantation for Conjunctival tumor. Clinico-pathologic Characteristics of Premalignant and Malignant Melanocytic Lesions of the Conjunctiva 19(3): 165-167, 145.
- Lezcano C, Jungbluth AA, Busam KJ (2021) PRAME Immunohistochemistry as an Ancillary Test for the Assessment of Melanocytic Lesions. Surgical Pathology Clinics 14(2): 165–175.
- Uguen A, Talagas M, Costa S, Duigou S, Bouvier S, et al. (2015) A p16-Ki-67-HMB45 Immunohistochemistry Scoring System as an Ancillary Diagnostic Tool in the Diagnosis of Melanoma. Diagnostic Pathology 10(1): 195.
- Dinehart MS, Dinehart SM, Sukpraprut Braaten S, High WA (2020) Immunohistochemistry Utilization in the Diagnosis of Melanoma. Journal of Cutaneous Pathology 47(5): 446-450.
- Ordóñez NG (2014) Value of melanocytic-associated Immunohistochemical Markers in the Diagnosis of Malignant melanoma: a Review and Update. Human Pathology 45(2): 191-205.
- Mihic Probst D, Mnich CD, Oberholzer PA, Seifert B, Sasse B et al. (2006) p16 Expression in Primary Malignant Melanoma Is Associated with Prognosis and Lymph Node Status. International Journal of Cancer 118(9): 2262–2268.
- Mihic Probst D, Kuster A, Kilgus S, Lesniewska BB, Heppner B et al. (2007) Consistent Expression of the Stem Cell Renewal Factor BMI-1 in Primary and Metastatic melanoma. International Journal of Cancer 121(8): 1764–1770.
- Brownstein S (2004) Malignant Melanoma of the Conjunctiva. Cancer Control 11(5): 310-316.
- Anastassiou G (2002) Prognostic Value of Clinical and Histopathological Parameters in Conjunctival mel-anomas: a Retrospective Study. British Journal of Ophthalmology 86(2): 163–167.
- Kenawy N, Lake SL, Coupland SE, Damato B (2013) Conjunctival Melanoma and Melanocytic intra-epi-thelial Neoplasia. Eye London England 27(2): 142-152.
- Jovanovic P, Mihajlovic M, Djordjevic Jocic J, Vlajkovic S, Cekic S et al. (2013) Ocular melanoma: an Overview of the Current Status. International Journal of Clinical and Experimental Pathology 6(7): 1230-1244.
- Shields CL (2011) Conjunctival Melanoma: Outcomes Based on Tumor Origin in 382 Consecutive Cases. Ophthalmology 118(2): 389-395.
- Esmaeli B (2001) Patterns of Regional and Distant Metastasis in Patients with Conjunctival Melanoma Experience at a Cancer Center over Four Decades. Ophthalmology 108(11): 2101–2105.
- Cohen VML, Tsimpida M, Hungerford JL, Jan H, Cerio R, et al. (2013) Prospective Study of Sentinel Lymph Node Biopsy for Conjunctival Melanoma. The British Journal of Ophthalmology 97(12): 1525-1529.
- Heindl LM, Hofmann Rummelt C, Adler W, Bosch J, Holbach L, (2011) et al. Prognostic Significance of Tumor-Associated Lymphangiogenesis in Malignant Melanomas of the Conjunctiva. Ophthalmology. 118(12): 2351-2360.
- Aziz H, Gastman BR, Singh AD (2015) Management of Conjunctival Melanoma: Critical Assessment of Sentinel Lymph Node Biopsy. Ocular Oncology and Pathology 1(4): 266–273.
- Mor JM, Rokohl AC, Koch K, Heindl LM (2019) Sentinel Lymph Node Biopsy in the Management of Con-junctival melanoma: Current Insights. Clinical Ophthalmology 13(12): 1297-1302.
- Barnhill RL, Lemaitre S, Lévy Gabrielle C, Rodrigues M, Desjardins L et al. (2016) Satellite in transit metastases in rapidly fatal con-junctival melanoma: implications for angiotropism and extravascular migratory metastasis (de-scription of a murine model for conjunctival melanoma). Pathology 48(2):166–176.
- Shields CL (2000) Conjunctival Melanoma: Risk Factors for Recurrence, Exenteration, Metastasis, and Death in 150 Consecutive Patients. Archives of Ophthalmology 118(11): 1497-1507.
- Kao A, Afshar A, Bloomer M, Damato B (2016) Management of Primary Acquired Melanosis, Nevus, and Conjunctival Melanoma. Cancer Control 23(2): 117–125.
- Esmaeli B (2011) Ophthalmic Oncology. Springer Science & Business Media.
- Anam K, Lazdun Y, Davis PM, Banas RA, Elster EA, et al. (2013) Amnion-Derived Multipotent Progenitor Cells Support Allo-graft Tolerance Induction. American Journal of Transplantation 13(6): 1416-1428.
- Grimes JM, Shah NV, Samie FH, Carvajal RD, Marr BP, et al. (2020) Conjunctival Melanoma: Current Treatments and Future Op-tions. American Journal of Clinical Dermatology 21(3): 371-381.
- Ikehata H, Ono T (2011) The Mechanisms of UV Mutagenesis. Journal of Radiation Research 52(2): 115-125.
- Rossi E, Schinzari G, Maiorano B, Pagliara M, Di Stefani A, et al. (2019) Conjunctival Melanoma: Genetic and Epigenetic Insights of a Distinct Type of Melanoma. International Journal of Molecu-lar Sciences 20(21): 5447.
- Yap YS, McPherson JR, Ong CK, Rozen SG, The BT, et al. (2014) The NF1 Gene Revisited – from Bench to Bedside. Oncotar-get 5(15): 5873-5892.
- Scholz SL, Cosgarea I, Süßkind D, Murali R, Möller I, et al. (2018) NF1 Mutations in Conjunctival Melanoma. British Journal of Cancer 118(9): 1243-1247.
- Cosgarea I, Ugurel S, Sucker A, Livingstone E, Zimmer L, et al. (2017) Targeted next Generation Sequencing of Mucosal Melanomas Identifies Frequent NF1 and RAS Mutations. Oncotarget (25): 40683–40692.
- Zhou R, Shi C, Tao W, Li J, Wu J, et al. (2019) Analysis of Mucosal Melanoma Whole-Genome Landscapes Reveals Clinically Relevant Genomic Aberrations. Clinical Cancer Research 25(12): 3548-3560.
- Krauthammer M, Kong Y, Bacchiocchi A, Evans P, Pornputtapong N, et al. (2015) Exome Sequencing Identifies Recurrent Mutations in NF1 and RASopathy Genes in sun-exposed Melanomas. Nature Genetics 47(9): 996-1002.
- Lally SE, Milman T, Orloff M, Dalvin LA, Eberhart CG, et al. (2022) Mutational Landscape and Outcomes of Conjunctival Melanoma in 101 Patients. Ophthalmology 129(6): 679-693.
- Gong HZ, Zheng HY, Li J (2018) The Clinical Significance of KIT Mutations in Melanoma. Melanoma Re-search 28(4): 259-270.
- Van Poppelen NM, Van Ipenburg JA, Van Den Bosch Q, Vaarwater J, Brands T, et al. (2021) Molecular Genetics of Conjunctival Melanoma and Prognostic Value of TERT Promoter Mutation Analysis. International Journal of Molecular Sciences 22(11): 5784.
- Zhang Y, Zhang Z (2020) The History and Advances in Cancer immunotherapy: Understanding the Char-acteristics of tumor-infiltrating Immune Cells and Their Therapeutic Implications. Cellular & Mo-lecular Immunology 17(8): 807-821.
- Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, et al. (2018) Encorafenib plus Binimetinib versus Vemurafenib or En-corafenib in Patients with BRAF-mutant Melanoma (COLUMBUS): a multicentre, open-label, Ran-domised Phase 3 Trial. The Lancet Oncology 19(5): 603-615.
- Sa HS, Daniel C, Esmaeli B (2022) Update on Immune Checkpoint Inhibitors for Conjunctival Melanoma. Journal of Ophthalmic and Vision Research 17(3): 405-412.
- Sagiv O, Thakar SD, Kandl TJ, Ford J, Sniegowski MC et al. (2018) Immunotherapy with Programmed Cell Death 1 Inhibitors for 5 Patients with Conjunctival Melanoma. JAMA Ophthalmology 136(11): 1236-1241.






























