Synthesis and Characterization of Biogenic Magnesium Oxide Nanoparticles as Antidiabetic Agents
Muhammad Basit Naeem1, Junaid Ahmad1, Bilal Arshad1, Rubace Fatima Mirza1, Rana Muhammad Sohail Afzal khan1, Muhammad Aetesam Nasir1, Shaharyar Ashraf1, Ifrah Saroosh1 and Muhammad Waqar Mazhar2*
1Department of medicine and surgery, Hitec-institute of medical sciences taxila cantt, Pakistan
2Department of Bioinformatics and Biotechnology, Government College University, Faisalabad, Pakistan
Submission: December 07, 2023; Published: December 19, 2023
*Corresponding Address: Muhammad Waqar Mazhar, Department of Bioinformatics and Biotechnology, Government College University, Faisalabad, Pakistan, Email: waqarmazhar63@gmail.com
How to cite this article: Muhammad Basit Naeem, Junaid Ahmad, Bilal Arshad, Rubace Fatima Mirza, Rana Muhammad Sohail Afzal khan, et al. Synthesis and Characterization of Biogenic Magnesium Oxide Nanoparticles as Antidiabetic Agents. Canc Therapy & Oncol Int J. 2024; 25(5): 556171. DOI:10.19080/CTOIJ.2024.25.556171
Abstract
The world has been persistently battling against diabetes for years. Besides all the advancements in therapeutic treatment, diabetes is still the fifth major death cause all over the world. The spread of diabetes has a growing trend due to ageing, changes in lifestyle, nutrition and lack of exercise. Several genetic elements and other behavioral, temporal and environmental factors may also contribute to the spread of this disease. Nanotechnology enabled the production of silver nanoparticles from natural materials that were potent inhibitors of α-glucosidase enzymes in the treatment of diabetes mellitus. MgO NPs showed strong interaction with the polar amino acids such as SER 30, ASP 37, and LYS 39 residues of α- glucosidase with the formation of hydrogen bonds. The specimen would be effective send safe with its cytotoxic concentration of 100 μg/mL. ZnO NPs represent a high binding relationship with the amino acids (ASP 46, GLU 286, and VAL 325) of α-glucosidase.
Keywords: Diabetes Mellitus; Nanopaticles; ZnO; α-glucosidase; Drugs
Introduction
An inherited disease caused by elevated blood glucose levels, or by the improper secretion of insulin hormone is called diabetes mellitus. Because of the defect, abnormal carbohydrate metabolism, and elevated blood glucose levels can lead to organ damage and immunological reactions that may affect pancreatic beta cell damage. The increased production of cytokine may lead to insulin resistance [1]. The world has been persistently battling against diabetes for years. Besides all the advancements in therapeutic treatment, diabetes is still the fifth leading death cause all over the world. The spread of diabetes is a growing trend due to aging, change in lifestyle, nutrition and lack of exercise. Several genetic elements and other behavioral, temporal and environmental factors may also contribute to the spread of this disease [2].
Diabetes is an endocrine system disease and according to the Global Systematic Search of published studies. According to the estimated ratio, 500 million people are affected by Type II diabetes in the surrounding world [3]. The prevalence rate is elevated among developed and under-developed countries and will tend to increase in all countries covered by the estimated period, but the highest diabetic ratio is observed in under-developed and developing countries [4]. Its prevalence is high in the adult population all over the world (4.7% in 2014 to 8.8% rise in 2017 and predicted to get elevated up to 9.9% in 2045) [5]. It is predicted by International Diabetes Federation (IDF) that by 2025, diabetes will affect 11.5 million people in Pakistan which made Pakistan the 5th most affected country on IDF classification of the diabetic population [3,6].
Advancement in the field of nanotechnology gains new insights into its various applications in treatment of many diseases counting diabetes mellitus [7]. Nanotechnology is an attractive field of research relating to the production of variable size nanoparticles, form and chemical composition, with controlled dispersion [8]. Biological, physical, and chemical approaches are being utilized for synthesizing nanoparticles [9]. In Biological methods, yeast extracts are used to produce nanoparticles which are more environment-favorable in comparison with the chemical and physical method [10]. Advancement in the field of research, yeast-based nanoparticles are used for the treatment of diabetes mellitus which are target specific and shows better efficiency and limited side effects due to the presence of the natural compounds and anti-diabetic activity [11]. Nanotechnology enabled the production of silver nanoparticles from natural materials that were potent inhibitors of α-glucosidase enzymes in the treatment of diabetes mellitus [12]. The glucose metabolizing enzymes α-glucosidase and α-amylase were inhibited by the produced silver nanoparticles from Allium cepa, resulting in a significant antidiabetic action [13,14]. The advancement of nanotechnology has allowed many materials including ZnO NPs are now being assessed for biological uses and disease-modifying therapies. According to this study, ZnO NPs can target different number of hallmarks of DM. As a result, ZnO NPs are a promising anti- diabetic agent that warrants further research and clinical experimentation [15-23] (Table 1).
Methodology
In-silico analysis
Protein Sequence Retrieval
The protein sequence of α-glucosidase from Saccharomyces cerevisiae was retrieved using NCBI by selecting the protein under all databases in navigation bar and enter α-glucosidase protein in search bar. α-glucosidase protein sequence was consisting of 584 amino acids under accession numberGAX66902.1.
3D structure prediction of α-glucosidase
The homology modeling approach was used to predict the 3D structure of α-glucosidase. For the selection of a suitable template the query sequence was used for Blastp analysis against pdb. The best query coverage of identified template sequence was 99% with 72% identity. The 3D structure of α-glucosidase was predict using MODELLER which is an offline software. Query sequence was pasted in Wint1.ali file before stearic and write the amino acids length which was 584. Align2d.py file was edit edited notepad++ and change the file name with target downloaded pdb file name which was ‘3a47’. Get-model.py file was edit with notepad++ and change the file name with ‘3a74’ in row 17. Starting and ending models were edited in row 20 and 21 with 1 and 5 respectively. Modeller was opened and write first command which was cd space and then paste the path of Modeller files. When the modeller detected the path then entered second command which was mod9.25 align2d.py. Two files were generated which were wnt1-3a47 and wnt1-3a47.pap. After generated both files third command was entered which was mod9.25 get-model.py which was generated Five models of protein.
Protein structure visualization
Protein structure was visualized by using chimera. Chimera determined the α and β-sheets of protein (α-glucosidase) and saved into pdb format [24].
Protein structure refinement
Refinement of 5 models of protein was done through GalaxyWEB. The GalaxyWEB server predicts protein structure from sequence by template- based modeling and refines loop or terminus regions.
α-glucosidase model evaluation: After the prediction of 5 models of α-glucosidase. The evaluation of the predicted models was done utilizing an online SAVES server. Under SAVES server different tools are present i.e. ERRAT [25] and RAMPAGE [26] which was compared the different parameters. ERRAT was helped to identify the quality factor of predicted proteins.
Ligand Preparation: The ligand (MgO NPs) was downloaded through PubChem. Then, the structure of MgO NPs was opened in chimera to save in pdb formatChemDraw 3D ultra was used to minimize the energy of ligand molecule.
AdmetSAR properties
The properties of ligand (MgO NP) identified by using admetSAR with input file as smile. This tool was helping to identify different properties of the ligand including Brain Blood Barrier (%), Human Intestinal Absorption (%), Biodegradation (%), Acute Oral Toxicity (%), Aqueous Solubility, Molecular Weight (g/Mol), Hydrogen Bond Acceptor, Hydrogen Bond Donor.
Molecular docking analysis
Α-glucosidase protein was docked with MgO NPs. The molecular docking analysis was performed using patchDock, which was helped to check the interactions between α-glucosidase and MgO NPs. The pdb format file of both ligand (MgO NPs) and protein (α-glucosidase) were uploaded under ligand and receptor molecule respectively using default parameters.
Results
3D structure prediction and evaluation
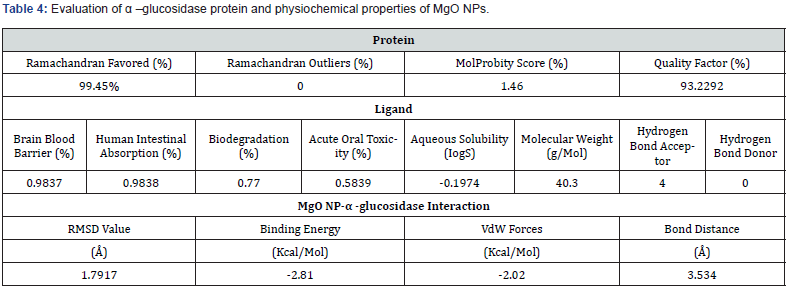
3D structure of the α-glucosidase protein was generated using Modeller. The α-glucosidase protein showed highly significant results after identifying the quality factor which was 93.22% through ERRAT, molprobity score was 1.46 %, and the physiochemical properties by using protparam, which predicted 68127.34 molecular weight with 5.59 theoretical PI. Total number of negatively and positively charged residues were also calculated such as Asp + Glu: 87, Arg + Lys:73 respectively. Aliphatic index 64.28 with grand average hydropathicity (GRAVY) -0.670 was also calculated. Protparam also identified that α-glucosidase protein is stable. Amino acids and atoms with their compositions were also measured as shown in table 1. 3D structure of α- glucosidase was also evaluated through verify 3D which measured 92.22 % score (Figures 1-4) (Tables 2 & 3).



α-glucosidase evaluation through rampage
RAMPAGE identified 99.45 % (581) amino acids in favored region. Three amino acids were calculated in the allowed region, such as PHE 177, ALA 278, GLU 530, and disallowed region contained no amino acids (Figure 4).



AdmetSAR properties of MgO NPs
AdmetSAR was used to identify the properties of MgO NPs (ligand) which includes brain blood barrier 0.9837 % with human intestinal absorption was 0.9838 %. The percentage of biodegradation of MgO NPs was also calculated 0.77 % with 0.589 % acute oral toxicity. The molecular weight of ligand (MgO NPs) was 40.3 g/mol with 4 hydrogen bond acceptor and no hydrogen bond donor was identified as shown in (Table 4).
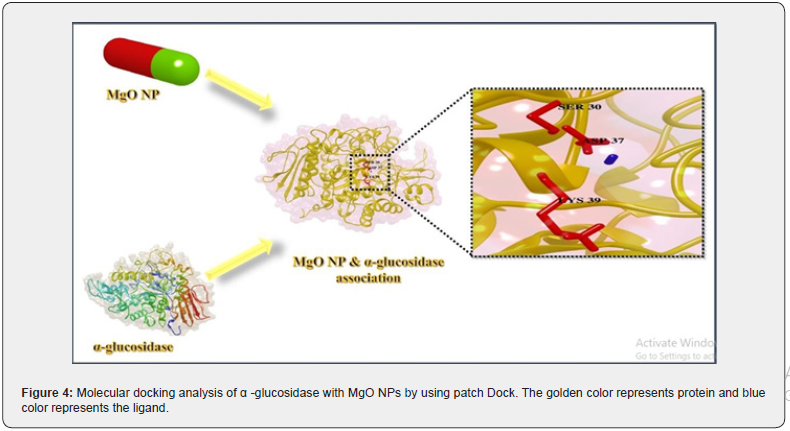
Molecular docking analysis of α-glucosidase with MgO NPs
α-glucosidase protein was docked with MgO NPs. The binding locations and size effect of MgO NPs on α-glucosidase association were found using docking analysis. Figure 4 represents the docking results of MgO NPs with α-glucosidase which showed conserved binding pocket. The docking complex has highest RMSD value (1.7917 Å), which is a good score. This association has the least binding energy of −2.81 kcal/Mol. The attractive VdW and the bond distance was also calculated which was -2.02 (kcal/Mol) and 3.534 Å respectively. This results indicate most facile interaction. Conserved residues of protein such as Serine (SER 30), Aspartic acid (ASP 37) and Lysine (LYS 39) were found, which showed highest interaction with the MgO NPs (Figure 4).
Discussion
Nanoparticles can be synthesized either through biological or chemical methods. The biological methods are cheap, reliable, environmentally friendly and easy, so they are gaining attention for the synthesis of nanoparticles and their beneficial role for mankind (Gahlawat & Choudhury, 2019). The biological interaction mainly depends on the crystallinity, shape, and size of the nanoparticles. On the other hand, the size and crystallinity of nanoparticles changed with calcination temperatures. (Nadaroglu et al., 2017; Shah et al., 2015) reported the synthesis of nanoparticles done by using biological organisms such as bacteria, actinobacteria, yeasts, molds, algae, and plants, or other products. Molecules in plants, and microbes, such as proteins, amines, enzymes, phenolic compounds, alkaloids, and pigments perform nanoparticles synthesis by reduction. In this research, the industrial yeast Saccharomyces cerevisiae was used to synthesize the monodispersed and more stable MgO NPs. Although the production of MgO NPs from fungal strain Aspergillus niger have previously been reported, the biogenic synthesis from yeast strain Saccharomyces cerevisiae has not been documented yet. There are several other associated advantages that have been observed in different functional groups conjugated with the surface of MgO NPs making it suitable for various biomedical applications (Ammulu et al., 2021) (Umaralikhan et al., 2018).
The physical confirmation of nanoparticles can be determined through various techniques. The intensity of the color of yeast culture increased due to the excitation of surface plasmon vibrations in the metal nanoparticles (Evanoff Jr & Chumanov, 2005). This significant observation indicates that the reduction of the Mg2+ ions take place extracellularly. The production and stability of the reduced MgO NPs in the colloidal solution were investigated using UV-spectrophotometer. Different functional groups, elements, morphology, shape, and size of the metal nanoparticles are determined through various techniques. By performing in-silico molecular docking and anti-diabetic assay (in-vitro) the activity of these nanoparticles could be better understood. In-silico molecular docking studies assess the best performance for MgO NPs which indicates the particle size of 32 nm will be appropriate for the optimum hole size of the enzyme (α- glucosidase). The docked models with negative binding energy for complex imply facile and favorable association of the nanoparticles and protein. MgO NPs showed strong interaction with the polar amino acids such as SER 30, ASP 37, and LYS 39 residues of α- glucosidase with the formation of hydrogen bonds. The specimen would be effective send safe below its cytotoxic concentration of 100 μg /mL. MgO NPs exposed in the present study are worthy entrants for α- glucosidase inhibition presenting a scarce selectivity. Hence, it is related to another research by (Prasad et al., 2019) that revealed, ZnO NPs represent a high binding relationship with the amino acids (ASP 46, GLU 286, and VAL 325) of α- glucosidase.
Acknowledgement
Conflict of interest: No potential conflicts of interest relevant to this article were reported.
Funding statement
Funding statement: No funds.
References
- De Kort S, Keszthelyi D, Masclee A (2011) Leaky gut and diabetes mellitus: what is the link? 12(6): 449-458.
- Cornelis MC, Hu Frank B (2012) Gene-environment interactions in the development of type 2 diabetes: recent progress and continuing challenges. 32: 245-259.
- Mazhar MW (2023) International Journal of Probiotics and Dietetics.
- Kaiser AB, Zhang N, Van Der Pluijm W (2018) Global prevalence of type 2 diabetes over the next ten years (2018-2028). Am Diabetes Assoc 67(Supp1).
- Iravani S (2011) Green synthesis of metal nanoparticles using plants. 13(10): 2638-2650.
- Mohammad FH, Nanji K (2018) Risk of Type 2 Diabetes among the Pakistani population: Results of a cross-sectional survey. Cureus 10(8): e3144.
- Balsells M, Apolonia García-Pa, Ivan Solà, Marta Roqué, Ignasi G, et al. (2015) Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ p. 350.
- Bamrungsap S, Zilong Z, Tao C, Lin W, Chunmei Li, et al. (2012) Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Nanomedicine 7(8): 1253-1271.
- Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9(6): 385-406.
- Ramya M, Subapriya M (2012) Green synthesis of silver nanoparticles. 1(1): 54-61.
- Saka R, Chella N (2020) Nanotechnology for delivery of natural therapeutic substances: a review. Environ Chem Letters 19(2): 1097-1106.
- Telrandhe R, Debarshi Kar M, Manish AK (2017) Bombax ceiba thorn extract mediated synthesis of silver nanoparticles: Evaluation of anti-Staphylococcus aureus activity. p. 376-379.
- Jini D, Sharmila S (2020) Green synthesis of silver nanoparticles from Allium cepa and its in vitro antidiabetic activity. Proceedings 22(3): 432-438.
- Mazher MW, Raza A, Sikandar M, Mahmood J, Saif S, et al. (2021) Just Role of Anti Diabetic Plants as Traditional Medicines. J Pharm Therap 9(3): 1137.
- San Tang K (2019) The current and future perspectives of zinc oxide nanoparticles in the treatment of diabetes mellitus. Life Sci 239: 117011.
- Manam DVK, Murugesan S (2013) Biogenic silver nanoparticles by Halymenia poryphyroides and its in vitro anti-diabetic efficacy. 5(12): 1001-1008.
- Sharma G, Soni R, Nakuleshwar D Jasuja (2017) Phytoassisted synthesis of magnesium oxide nanoparticles with Swertia chirayaita. J Taibah Uni Sci 11(3): 471-477.
- Noor S, Ziaullah S, Aneela J, Amjad A, Syed Bilal H, et al. (2020) A fungal based synthesis method for copper nanoparticles with the determination of anticancer, antidiabetic and antibacterial activities. J Microbiol Methods 174: 105966.
- Senthilkumar P, Lakshmi P, Ranjith Santhosh Kumar DS, Bhuvaneshwari J, Prakash (2015) Potent α-glucosidase inhibitory activity of green synthesized gold nanoparticles from the brown seaweed Padina boergesenii. Int J Recent Adv Multidisplinary Res 2(11): 917-923.
- Veeramani S, Arya PN, Kousika Y, Ramachandran S, Arivalagan P, et al. (2022) Nigella sativa flavonoids surface coated gold NPs (Au-NPs) enhancing antioxidant and anti-diabetic activity. Process Biochemistry 114: 193-202.
- Popli D, Vishaka A, Akshatha BS, Namratha MN, Ranjitha VR, et al. (2018) Endophyte fungi, Cladosporium species-mediated synthesis of silver nanoparticles possessing in vitro antioxidant, anti-diabetic and anti-Alzheimer activity. Artif Cells Nanomed Bitechnol 46(Sup1): 676-683.
- Velsankar K, Suganya S, Muthumari P, Mohandoss S, Sudhahar S, et al. (2021) Ecofriendly green synthesis, characterization and biomedical applications of CuO nanoparticles synthesized using leaf extract of Capsicum frutescens. 9(5): 106299.
- Arvanag FM, Abolfazl B, Aziz Habibi-Y, Shima Rahim P (2019) A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L seed extract. Mater Sci Eng C Mater Biol Appl 97: 397-405.
- Goddard TD, Huang CC, Thomas EF (2007) Visualizing density maps with UCSF Chimera. J Struct Biol 157(1): 281-287.
- Ravi S, Punabaka J, Bhasha S, Ganjikunta Venkata S, Kesireddy Sathyavelu Reddy (2018) Identification of Anticancer Lead Molecules against PRR11 Protein Target with Combination of Protein Modelling Through Threading Approach, Structure Based Chemical Screening of ZINC Database and Pharmacokinetic Properties. Indian J Pharmaceutical Education Res 52(3): 381-388.
- Dhal AK, Alok P, Rajani Kanta M, Soon-Il Yun (2019) An immuno informatics approach for design and validation of multi-subunit vaccine against Cryptosporidium parvum 224(6): 747-757.






























