“Impact of Depth of Invasion on Outcome of Operated Oral Cavity Carcinoma in Terms of Locoregional recurrence after Adjuvant Treatment”
Dr. Poonam Gupta*
Sr. Consultant, Department of Radiation Oncology, India
Submission: January 16, 2023; Published: January 26, 2023
*Corresponding Address: Dr. Poonam Gupta, Sr. Consultant, Department of Radiation Oncology, HPPCH Gorakhpur, India
How to cite this article: Dr. Poonam G. “Impact of Depth of Invasion on Outcome of Operated Oral Cavity Carcinoma in Terms of Locoregional recurrence after Adjuvant Treatment”. Canc Therapy & Oncol Int J. 2023; 23(1): 556105. DOI:10.19080/CTOIJ.2023.23.556105
Abstract
Background: Several histopathologic factors in the primary lesion are associated with adverse prognosis. Tumor thickness and depth of invasion have been shown to confer a higher risk of regional metastases. Perineural invasion has been correlated with cervical lymph node metastases, extracapsular extension, and diminished survival. This study is aimed to find the association of depth of invasion with locoregional control.
Material & Methods: This retrospective study was performed in 140 patients of carcinoma of oral cavity in Hanuman Prasad Poddar Cancer Hospital and Research Centre-Gorakhpur-India from 2018 to 2022. All the patients underwent surgery and adjuvant radiation with or without chemotherapy (according to indication).
Results: All patient had oral carcinoma with particular subsite including tongue, alveolus, lip and buccal mucosa. Among them all were male except 4 were female .After surgery they all received adjuvant treatment .The follow up period was from completion of radiation till november2022.
Conclusion: The depth of invasion is very strong predictor in locoregional control of oral cavity carcinoma. More the depth of invasion there is more chances of lymph nodal metastasis and decrease in survival.
Keywords: ENE: Extracapsular Extension; PNI: Perineural Invasion; END: Elective Lymph Node Dissection
Introduction
There are two clinical pathways for the management of oral cavity carcinoma: one pathway for rly-stage disease and another for locally advanced disease. The NCCN guidelines recommend surgery as the preferred treatment approach for early-stage T1 orT2N0 lesions of the oral cavity, for more advanced disease (T3/T4a, N0 or T1/T4a, N1/N3), NCCN guidelines recommend a combined modality approach involving surgery followed by adjuvant radiation or chemoradiation. The functional outcomes after primary surgical management are acceptable due to advances in microvascular reconstruction techniques. Nevertheless, definitive radiotherapy may be a treatment option for patients who are not candidates for surgery because of medical reasons or who refuse surgery. Multidisciplinary approach is paramount in the evaluation and management of patients with oral cavity cancer. There has been recent interest in postoperative chemoradiation for patients with high-risk pathologic features. The impact of chemoradiotherapy appears to be most pronounced in patients with ENE and or microscopically involved surgical margins. The most significant prognostic factor for outcome in oral cavity carcinoma is the presence of cervical metastases. In patients with positive cervical metastases, the 5-year survival is reduced by approximately 50% compared to those without cervical metastases. The prognosis diminishes further when patients harbor multiple levels of nodal involvement or ENE. Several histopathologic factors in the primary lesion are associated with adverse prognosis. Tumor thickness and depth of invasion have been shown to confer a higher risk of regional metastases. Perineural invasion has been correlated with cervical lymph node metastases, extracapsular extension, and diminished survival. Microvascular invasion has also been correlated significantly with cervical lymph node metastases. However, lymphatic invasion has not been correlated significantly with cervical lymph node invasion. The prognostic significance of grade has also been evaluated. Because of the wide variation in pathologic interpretation, it is difficult to discern the independent value of histologic grading as a prognostic or predictive value.
Materials & Method
This retrospective study was performed in 140 patients of carcinoma of oral cavity in Hanuman Prasad Poddar Cancer Hospital and Research Centre-Gorakhpur-India from 2018 to 2022.All the patients underwent surgery and adjuvant radiation with or without chemotherapy (according to indication). All the patients received radiation dose of 60 Gy in 30# with or without chemotherapy inj cisplatin 40mg/m2. The follow up period was from completion of radiation to November 2022.
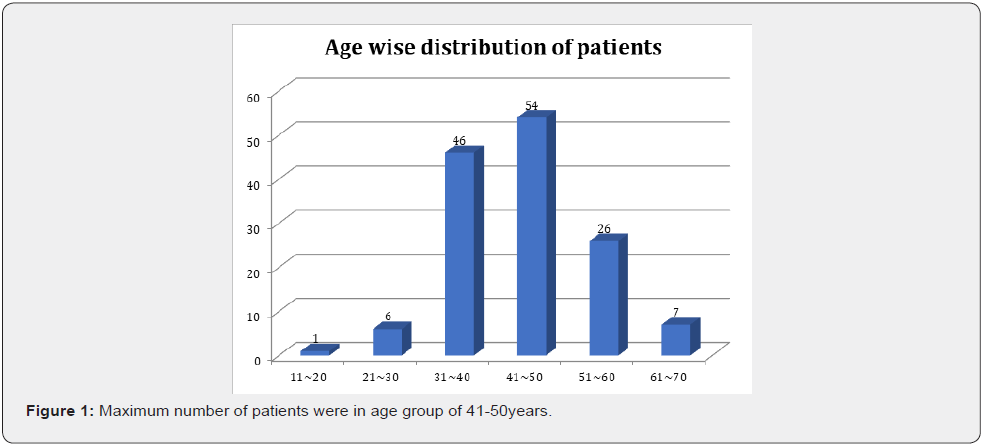
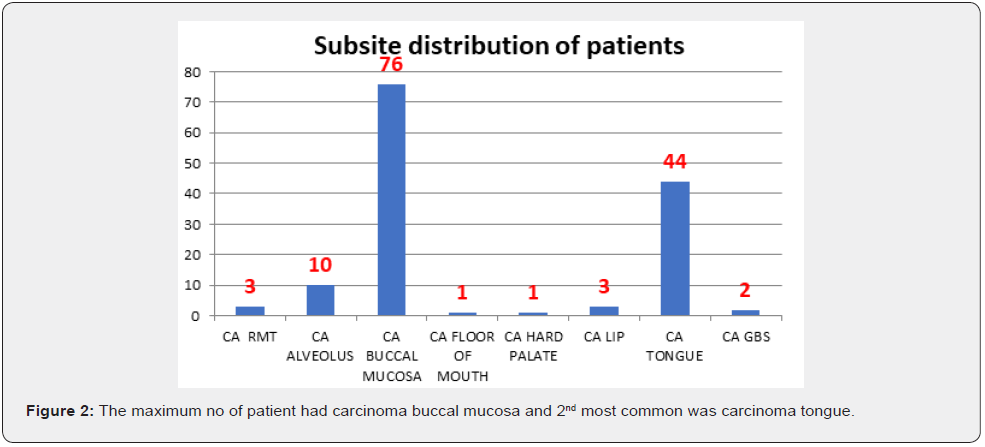
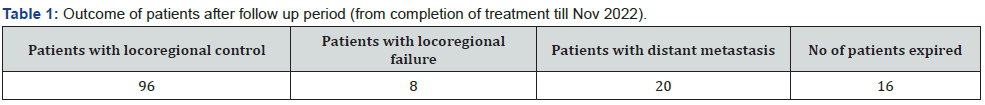
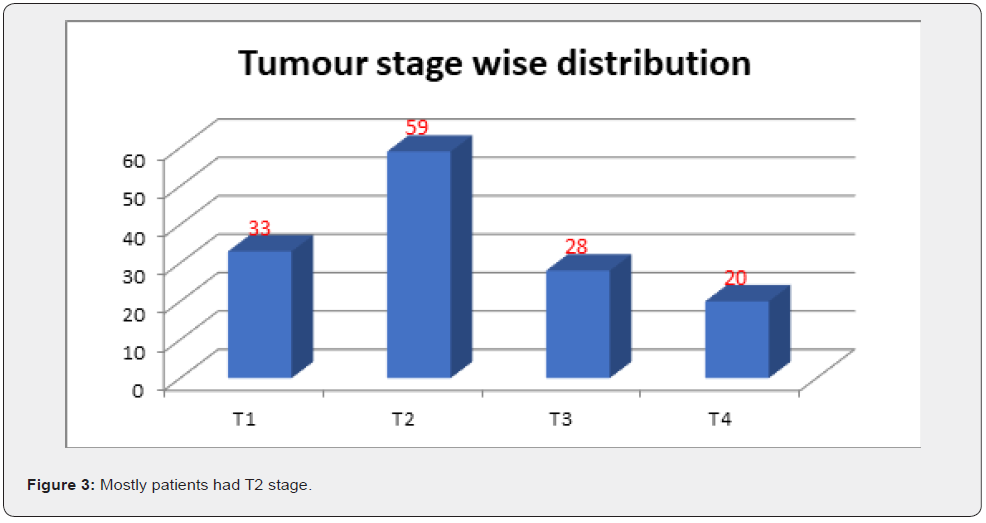
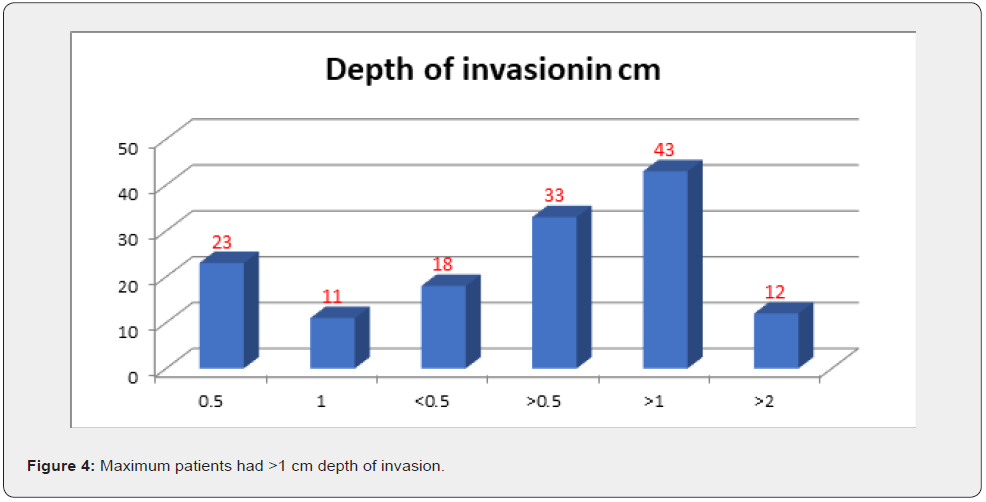
Results
After analysis of 140 patients with oral cavity cancer different results found (Figures 1-4) (Table 1).
i. The patients who had poor outcome were with high-risk features such as advanced T stage , more depth of invasion than 0.5cm, more no of lymph node metastasis and ENE.
ii. The patients with more depth of invasion had more no of cervical node positive in their post op histopathology.
Discussion
The most significant prognostic factor for outcome in oral cavity carcinoma is the presence of cervical metastases. In patients with positive cervical metastases, the 5-year survival is reduced by approximately 50% compared to those without cervical metastases. The prognosis decreases further when patients have multiple levels of nodal involvement or ENE(extranodal extension). Tumor size and depth of invasion are currently the most reliable indicators for predicting cervical metastases in patients with oral tongue squamous cell carcinoma [2-5]. Because of the high risk of nodal metastases, the neck should be addressed either with surgery or radiation in all but the earliest tumors of the oral tongue. Patients with small oral tongue cancers should be considered for neck therapy, particularly if the primary tumor exhibits extension onto the floor of the mouth or there is increased tumor thickness. Treatment of the clinically negative neck is most often accomplished by supraomohyoid neck dissection. END appears to result in better overall cancer outcome than observation. Potential pitfalls of observation include a salvage rate of only one-third for patients who do not undergo END along with resection of the oral cavity primary [6-8].
Conclusion
Advanced stage oral cavity carcinoma has poor outcome even after multimodality treatment approach. The greater the depth of invasion along with other high-risk factors such as large tumor stage, ENE, PNI and more no of involvement of nodes. The depth of invasion could be a prognostic factor with other associated highrisk. factors.
References
- Chen AY, Myers JN (2001) Cancer of the oral cavity. Dis Mon 47(7): 275-361.
- Myers JN, T Elkins, D Roberts, R M Byers (2000) Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg 122(1): 44–51.
- Johnson JT, E N Myers, V L Schramm Jr, D Borochovitz, B A Sigler (1981) The extracapsular spread of tumors in cervical node metastasis. Arch Otolaryngol 107(12): 725–729.
- Borges AM, Shrikhande SS, Ganesh B (1989) Surgical pathology of squamous carcinoma of the oral cavity: its impact on management. Semin Surg Oncol 5(5): 310–317.
- Soo KC, R L Carter, C J O'Brien, L Barr, J M Bliss, et al. (1986) Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope 96(10): 1145–1148.
- Lozza L, A Cerrotta, G Gardani, M De Marie, A Di Russo, et al. (1997) Analysis of risk factors for mandibular bone radionecrosis after exclusive low dose-rate brachytherapy for oral cancer. Radiother Oncol 44(2): 143-147.
- Pignon JP, Aurélie le Maître, Emilie Maillard, Jean Bourhis; MACH-NC Collaborative Group et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92(1): 4–14.
- Patil VM, K Prabhash, V Noronha, A Joshi, V Muddu et al. (2014) Neoadjuvant chemotherapy followed by surgery in very locally advanced technically unresectable oral cavity cancers. Oral Oncol 50(10): 1000–1004.






























