Eugenic and Matriclinous-BRCA Associated Carcinoma Breast
Anubha Bajaj*
Histopathologist in A B Diagnostics, New Delhi, India
Submission: December 06, 2022; Published: January 23, 2023
*Corresponding Address: Anubha Bajaj, Histopathologist in A B Diagnostics, New Delhi, India
How to cite this article: Anubha B. Eugenic and Matriclinous-BRCA Associated Carcinoma Breast. Canc Therapy & Oncol Int J. 2023; 23(1): 556104. DOI:10.19080/CTOIJ.2023.23.556104
Mini Review
BRCA associated carcinoma breast emerges within subjects demonstrating a concomitant BRCA germline mutation. Neoplasm frequently manifests as a high grade, invasive ductal carcinoma breast of no special type (NST). Generally, BRCA associated carcinoma breast is accompanied by BRCA1 germline mutation wherein BRCA1 associated breast cancer is devoid of cogent classification by World Health Classification (WHO). Tumefaction is additionally designated as BRCA1 related breast cancer or BRCA1 germline mutation related breast cancer [1,2]. BRCA1 germline mutation is observed in ~3% breast carcinomas. In contrast to sporadic carcinoma breast, BRCA associated carcinoma enunciates significantly diverse histopathological and molecular characteristics. Tumefaction exhibits prominent lymphocytic infiltrate and a ‘pushing’ tumour perimeter [1,2]. Generally, neoplasm is immune non-reactive to oestrogen receptors (ER), progesterone receptors (PR) and HER2neu and is designated as ‘triple negative’ whereas molecular neoplastic manifestation is basal-like and of intrinsic subtype [1,2]. BRCA associated carcinoma breast frequently exemplifies a hereditary predisposition, a factor which contributes to disease emergence. Around ~25% of BRCA associated carcinoma breast occur as a component of hereditary carcinoma breast [1,2]. Majority (60%) of incriminated subjects delineate emergence of BRCA associated carcinoma breast or carcinoma ovary by 70 years. Cogent genetic and environmental factors can alter proportionate emergence of carcinoma breast with accompanying BRCA1 germline mutations [1,2]. In contrast to sporadic or non BRCA1 / BRCA2 carcinoma breast, factors predicting disease emergence appear as age of disease onset < 50 years and immune non reactivity to HER2neu, oestrogen receptors (ER) and progesterone receptors (PR) [1,2].
BRCA1 is a tumour suppressor gene situated upon chromosome 17q21 and appears as an enlarged gene comprised of 5592 nucleotides which is prone to germline mutations across the entire gene. Hot spot mutations are generally absent. Majority (80%) of mutations induce a premature stop codon [1,2]. Majority (> 80%) of associated carcinomas breast may depict BRCA1 germline mutation carriers. Besides, wild type allele may disappear due to loss of heterozygosity (LOH) [1,2]. BRCA1 protein is critical in generating appropriate reaction to DNA injury, especially double strand break repair of DNA by homologous recombination. BRCA1 deficiency induces defective repair of DNA double strand break with consequent chromosomal instability and expedited tumorigenesis [1,2]. Deficit of p53 gene is an essential criterion for genesis of carcinoma breast in subjects with BRCA1 germline mutation carriers. In contrast to sporadic carcinoma breast, around 40% of BRCA1 associated carcinoma breast demonstrate a p53 genetic mutation [1,2]. BRCA2 is a tumour suppressor gene situated upon chromosome 13q12-13 [1,2]. BRCA associated invasive carcinoma breast exhibits a specific pattern of chromosomal gains and losses as detected by array comparative genomic hybridization (CGH) or multiplex PCR (MLPA) analysis.
Requisite genetic testing is recommended in subjects with recently detected carcinoma breast or high-risk neoplasms contingent to criterion defined by National Comprehensive Cancer Network (NCCN). BRCA1 germline mutations can be discerned with DNA sequencing analysis of breast neoplasms [1,2]. Roughly 25% carriers of BRCA1 and BRCA2 genetic mutation may be overlooked with contemporary genetic analysis [1,2]. Characteristically, subjects < 50 years are implicated as BRCA associated carcinoma breast enunciates an antecedent disease emergence. The neoplasm is commonly delineated in specific population groups or individuals depicting a family history of carcinoma breast and carcinoma ovary or certain infrequently discerned malignancies. Generally, neoplasm is immune non-reactive to oestrogen receptors, progesterone receptors and HER2 neu (triple negative) [1,2]. Upon microscopic examination, high grade, invasive ductal carcinoma is commonly expounded. Tumefaction exhibits abundant aggregates of intra-tumour and peritumoral lymphocytes. Alternatively, invasive breast carcinoma with medullary features is frequently exemplified [1,2] (Figures 1 & 2) (Table 1).
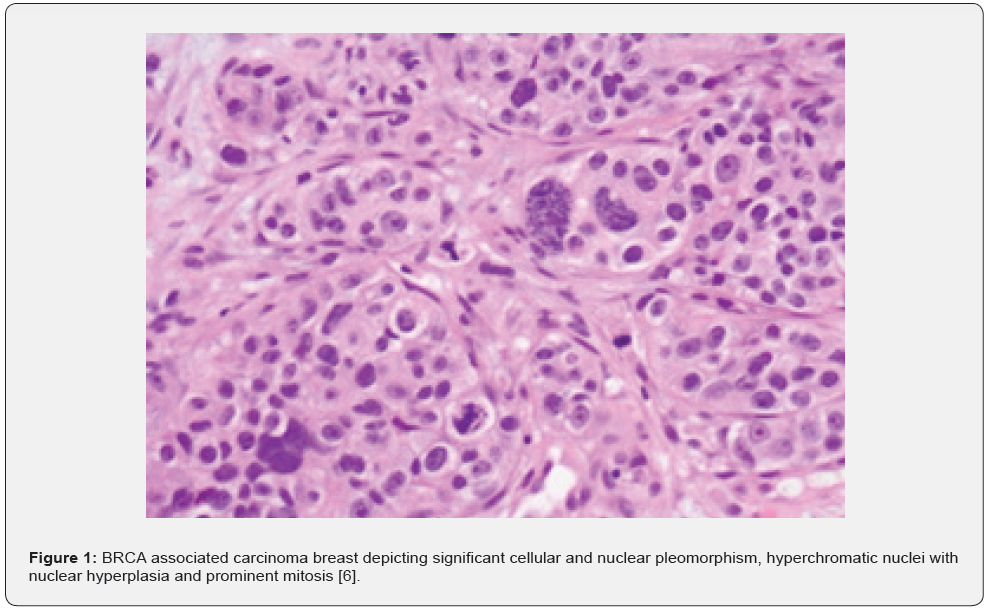
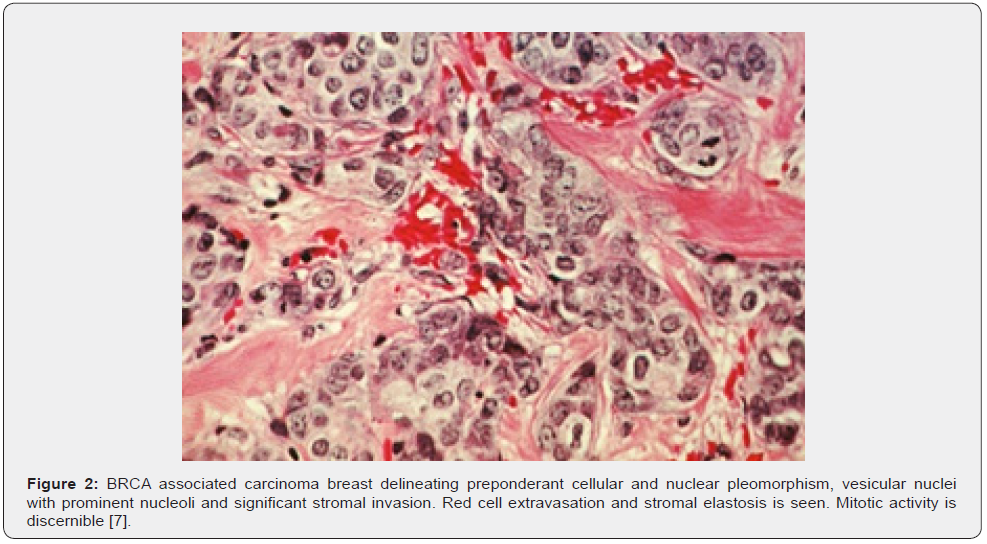
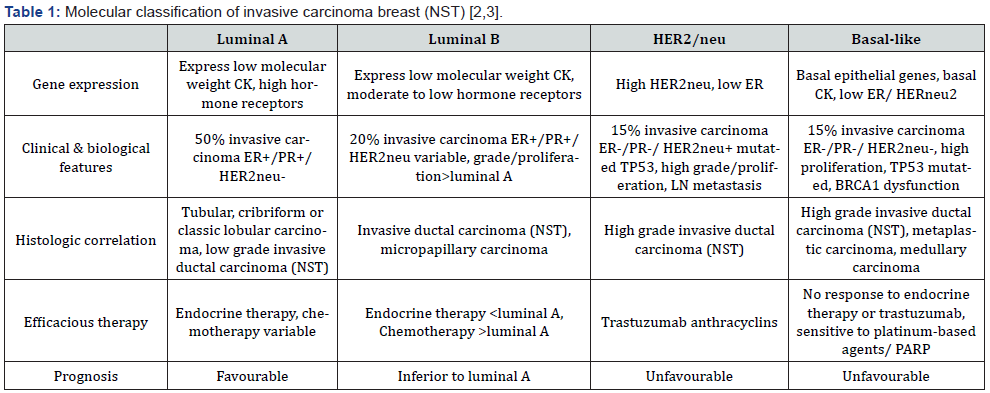
ER- oestrogen receptor, PR-progesterone receptor, CK-cytokeratin, NST-no special type, LN- lymph node PARP-poly-adenosine diphosphate ribose polymerase.
BRCA associated carcinoma breast is immune reactive to basal or myoepithelial cell markers as CK5/6, CK14, EGFR, hypoxia related proteins as HIF1a, GLUT1, CAIX or cell cycle related proteins as cyclin A, cyclin B1 or cyclin E. Ki67 proliferation index is elevated [3-5]. The neoplasm is immune non-reactive to oestrogen receptors, progesterone receptors, HER2neu, oestrogen receptor associated genes as BCL2, p27, cyclin D1, p21, p16, CD4, luminal cell markers as CK8/18 and variably immune non-reactive to BRCA1 [4,5]. BRCA associated carcinoma breast requires segregation from invasive carcinomas breast emerging from various mechanisms of BRCA deficiency as BRCA1 somatic mutation or BRCA1 hyper-methylation and high-grade triple negative invasive ductal carcinoma (NST) devoid of BRCA1 germline mutations [1,2].
Pre-symptomatic carriers of BRCA1 genetic mutation can be subjected to prophylactic mastectomy, a manoeuver which decimates possible neoplastic emergence by > 90% [4,5]. Symptomatic subjects delineating invasive carcinoma breast can be treated with mastectomy which represents as a contraindication to breast conservation therapy. However, prognostic outcomes of aforesaid surgical manoeuvers appear identical [4,5]. Neoplasm exhibits significant sensitivity to pertinent chemotherapy.
Prognostic outcomes of neoplasms associated with BRCA1 genetic status remain debatable [4,5].
References
- Casaubon JT, Kashyap S et al. (2022) BRCA 1 and 2. Stat Pearls International 2022, Treasure Island, Florida, USA.
- Aitmagambetova MA, Smagulova GA, Rustem R Tuhvatshin, Azhar N Zheksenova, Ainur Amanzholkyzy, et al. (2022) Genetic and clinical characteristics of BRCA-associated hereditary breast cancer in the West region of Kazakhstan. Carcinogenesis 43(9): 838-841.
- Eliyatkın N, Yalçın E, Baha Zengel, Safiye Aktas , Enver Vardar, et al. (2015) Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J Breast Health 11(2): 59-66.
- Valencia OM, Samuel SE, Rebecca K Viscusi, Taylor S Riall, Leigh A Neumayer, et al. (2017) The Role of Genetic Testing in Patients With Breast Cancer: A Review. JAMA Surg 152(6): 589-594.
- Daly MB, Pilarski R, Michael Berry, Saundra S Buys, Meagan Farmer, et al. (2017) NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J Natl Compr Canc Netw 15(1): 9-20.
- Image 1 Courtesy: Pathology outlines.
- Image 2 Courtesy: Institute of cancer research.






























