The Skeletal Internecine - Osteogenic Sarcoma
Anubha Bajaj*
Histopathologist in A B Diagnostics, New Delhi, India
Submission: November 25, 2022; Published: January 19, 2023
*Corresponding Address: Anubha Bajaj, Histopathologist in A B Diagnostics, New Delhi, India
How to cite this article: Anubha B. The Skeletal Internecine -Osteogenic Sarcoma. Canc Therapy & Oncol Int J. 2023; 23(1): 556103. DOI:10.19080/CTOIJ.2023.23.556103
Mini Review
Osteosarcoma is a commonly discerned, high-grade sarcoma of skeletal system or a primary, solid bone tumour wherein the malignant tumefaction expounds a capacity to synthesize bone. Contingent to localization, osteosarcoma depicts diverse variants situated within the bone or upon bony surface with varying tumour grades denominated as low grade, intermediate grade, or high-grade tumefaction. Majority (> 90%) of osteosarcomas emerge as conventional, high grade, intramedullary neoplasms. Parosteal osteosarcoma is a frequently delineated surface osteosarcoma. Low grade central osteosarcoma is infrequently discerned and represents ~2% of osteosarcoma whereas high grade osteosarcoma manifests <1% of neoplasms [1,2]. In contrast to parosteal osteosarcoma, periosteal osteosarcoma is exceptionally discerned. Besides, extra-skeletal osteosarcoma is an exceptional disease emerging in adults [1,2].
Microscopic examination of osteosarcoma depicts a typical filigree-like pattern. Upon radiographic examination, osteosarcoma represents as an aggressive neoplasm. Therapeutic strategies and prognostic outcomes are contingent to tumour grade, response to neoadjuvant chemotherapy and emergence of tumour metastasis [1,2]. Osteogenic sarcoma is categorized as ~primary osteosarcoma where osteosarcoma emerges within a normal bone ~secondary osteosarcoma where osteosarcoma arises within bone altered due to factors such as preceding radiation therapy, coexisting Paget’s disease of bone or bone infarction [1,2].
Conventional, high-grade osteosarcoma delineates morphologic subtypes which may coexist within a singular neoplasm as ~osteoblastic osteosarcoma demonstrating abundant synthesis of bone matrix ~chondroblastic osteosarcoma wherein the cartilaginous matrix is comprised of high-grade cartilage ~fibroblastic osteosarcoma demonstrating foci of neoplastic spindle-shaped cells [1,2]. •Parosteal osteosarcoma is a low-grade osteosarcoma situated upon cortical bone surface. Low grade central osteosar coma is a low-grade neoplasm arising within medullary cavity of bone. Periosteal osteosarcoma is an intermediate grade, malignant neoplasm configuring bone and cartilage confined to cortical surface of bone or demonstrating incrimination of periosteum. High grade surface osteosarcoma is a high-grade tumefaction arising from cortical surface of bone. Extra-skeletal osteosarcoma is a malignant bone neoplasm emerging within circumscribing soft tissue and may demonstrate chondroblastic or fibroblastic differentiation although additional pathways of tumour differentiation are absent. Majority of extra-skeletal lesions appear as primary neoplasms whereas few secondary tumefactions may appear after radiation therapy [1,2].
Osteosarcoma displays a bimodal age of disease emergence. Majority of primary osteosarcomas appear between 10 years to 14 years although secondary osteosarcoma occurs in adults > 40 years [1,2]. Conventional, high grade, intramedullary osteosarcoma enunciates a complex karyotype. Inactivation of TP53 is observed. IDH1 or IDH2 genetic mutations are usually absent. Occurrence of Li Fraumeni syndrome enhances possible emergence of osteosarcoma. Inactivation of Rb gene is encountered wherein germline mutations within RB1 augments possible emergence of post-radiation osteosarcoma. Amplification or gain of chromosome 8q21-24 with constituent c-Myc is encountered. Genetic amplification of MDM2 may ensue [1,2]. Parosteal and low-grade central osteosarcoma exemplifies genetic amplification of MDM2 situated upon chromosome 12q13-15 along with mutation of TP53 gene. Neoplastic karyotype exhibits minimal anomalies although supernumerary ring chromosomes are delineated [1,2]. Osteosarcoma commonly incriminates long bones of extremities abutting proliferative growth plates as distal femur, proximal tibia or proximal humerus although no bone of tumour emergence is exempt. Bony metaphysis, diaphysis or epiphysis are implicated in descending order of frequency. Tumour incrimination of jaw, pelvis or vertebral column may ensue in elderly subjects [1,2].
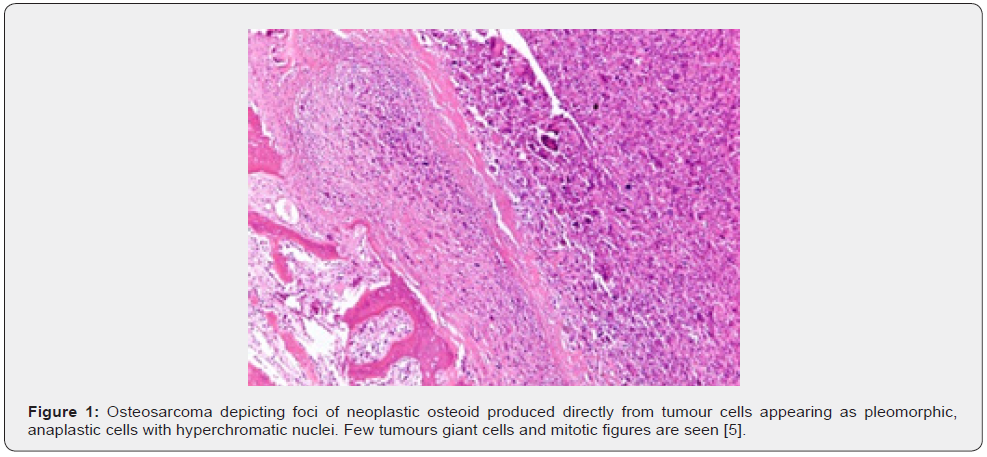
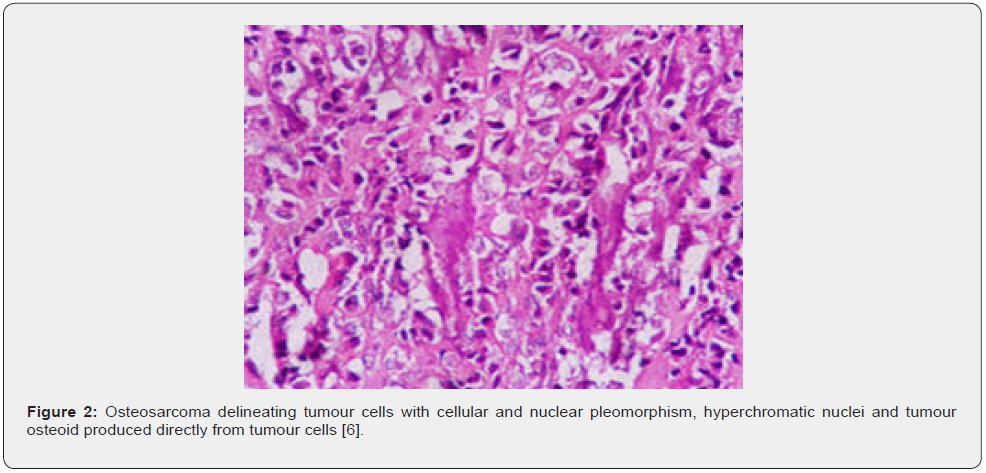
Osteosarcoma is exceptionally discerned within small bones of hands and feet. Conventional, multifocal osteosarcoma may concur with Paget’s disease of bone, predominantly the polyostotic variant [1,2]. Parosteal osteosarcoma generally emerges upon cortical bone surface and incriminates metaphysis. Commonly, neoplasm is discerned within distal posterior femur, proximal tibia or proximal humerus. Tumefaction infrequently arises within flat bones [1,2]. Low grade central osteosarcoma arises within long bones as distal femur or proximal tibia. Metaphysis or diaphysis are implicated although neoplasm may occupy medullary space of entire bone. Tumour emergence within flat bones or small tubular bones of hands and feet is uncommon [1,2]. Periosteal osteosarcoma arises from periosteum. Generally, neoplasm originates within metaphyseal or meta-diaphyseal region and frequently demonstrates an anterior or medial epicentre. Tumefaction may encase incriminated bone. Commonly, long bones or pelvis are involved. Tumefaction is infrequently discerned within clavicle, ribs, cranial bones, or jaw.
High grade surface osteosarcoma may incriminate cortical surface of long bones as femur, tibia, or humerus. Extra-skeletal osteosarcoma frequently incriminates deep soft tissues of thigh, buttocks, shoulder girdle, trunk, or retroperitoneum. Cutaneous surfaces or subcutaneous tissue may demonstrate tumour emergence in ~ 10% subjects [1,2]. Generally, osteosarcoma occurs as a painful, enlarging tumour mass. Few (~10%) instances may depict emergence of pathological fracture. Upon gross examination, high grade, intramedullary osteosarcoma emerges as an intramedullary tumefaction with metaphyseal epicentre, cortical permeation and tumour extension into surrounding soft tissue which elevates the periosteum. Mean tumour magnitude varies between 5 centimetres to 10 centimetres [1,2]. Cut surface is gritty, hard, or mineralized. Foci of haemorrhage, necrosis and cystic change may occur. Cartilaginous zones may be exemplified within chondroblastic osteosarcoma [1,2].
Parosteal osteosarcoma represents as a hard, lobulated tumefaction adherent to cortex. Cartilaginous nodules partially superimposed upon tumour surface or dedifferentiated foci of soft, fleshy tissue are variably discerned [1,2]. Low grade central osteosarcoma frequently permeates entire metaphysis and diaphysis of incriminated bone. Cut surface is firm and gritty. Neoplasm may demonstrate focal cortical destruction, periosteal reaction, and infiltration of soft tissue [1,2]. Periosteal osteosarcoma exhibits broad based, sessile tumour nodules emerging from cortical surface of bone. Besides, tumour may circumferentially incriminate bone. Bony cortex is thickened and delineates a scalloped extraneous surface. Base of tumour is significantly ossified. Tumour surface can be predominantly cartilaginous. Calcified spicules appear to extend perpendicularly from bone cortex into tumour mass and intermingle with superimposed cartilage. Cortical permeation and medullary extension of tumour is exceptional [1,2]. High grade surface osteosarcoma is composed of tumour emerging from cortical surface with erosion and invasion of cortex. Cut surface may appear as osteoblastic, chondroblastic or fibroblastic. Focal tumour necrosis is observed.
Occurrence of osteosarcoma following neoadjuvant chemotherapy requires dissection along long axis and tumour mapping [1,2]. Conventional, high grade, intramedullary osteosarcoma exhibits tumour progression with permeation, replacement of medullary spaces, circumscription, and erosion of native bone trabeculae along with impaction of Haversian system by neoplastic cells. Cortical destruction with soft tissue tumour infiltration may ensue. Neoplastic cells display significant pleomorphism, cellular and nuclear atypia and hyperchromatic nuclei [1,2]. Singular neoplasm enunciates divergent cellular morphologies as epithelioid, plasmacytoid, spindle-shaped, miniature round cells, clear cells, or tumour giant cells. Neoplastic bone or tumour osteoid emerging directly from tumour cells is a pathognomonic feature. Tumefaction commonly represents with filigree or lace-like, disorganized, woven bone which appears contiguous with neoplastic cells. Broad sheets of bone are configured [1,2].
Foci of normalization with neoplastic cells demonstrating decimated cytological atypia entrapped within bone matrix can be discerned. Scaffolds or appositional deposition of neoplastic osteoid upon native bone trabeculae are enunciated [1,2]. Non neoplastic giant cells appear intermingled with neoplastic cells. Contingent to predominantly secreted matrix, conventional osteosarcoma depicts distinctive morphological subtypes, devoid of pertinent prognostic significance designated as osteoblastic, chondroblastic or fibroblastic variants which may appear commingled within a singular neoplasm [1,2]. ~Osteoblastic osteosarcoma predominantly displays a matrix of neoplastic bone ~Chondroblastic osteosarcoma predominantly depicts a matrix of high grade, neoplastic cartilage ~Fibroblastic osteosarcoma is composed of spindle-shaped or epithelioid cells demonstrating severe atypia. Tumour cells may secrete minimal or copious amounts of extracellular collagen. Telangiectatic osteosarcoma expounds a multi-loculated neoplasm demonstrating enlarged, blood filled spaces admixed with high grade neoplastic cells and neoplastic bone amalgamated within traversing fibrous tissue septa, akin to aneurysmal bone cyst [1,2].
Additionally, morphological subtypes as epithelioid variant, osteoblastoma-like variant, chondroblastoma-like variant, chondromyxoid fibroma-like osteosarcoma, clear cell variant, small cell variant or giant cell rich variant demonstrating numerous osteoclast-like giant cells may be exemplified [1,2]. Osteosarcoma emerging secondary to Paget’s disease occurs as a high grade, intramedullary osteosarcoma, is permeated with innumerable osteoclast-like giant cells and may simulate diverse variants of osteosarcoma [1,2]. Parosteal osteosarcoma may be hypo-cellular and exhibits tumour invasion into circumscribing soft tissue or cortical or medullary bone. Neoplastic cells appear as fibroblast- like, spindle-shaped cells with minimal atypia and permeate bony trabeculae. Mitotic figures appear scattered. Neoplastic bone configures parallel bony trabeculae rimmed with osteoblasts. Cartilaginous component is frequently discerned. Tumefaction may depict hyper-cellular nodules. Partially superimposed cartilaginous cap is moderately cellular with disarranged chondrocytes demonstrating mild to moderate atypia [1,2]. Dedifferentiation may occur in ~25% instances with abrupt transformation into high grade sarcoma. High grade osteosarcoma exemplifies as high grade, undifferentiated pleomorphic sarcoma. Fibro-sarcoma is commonly encountered within reoccurring neoplasms although primary tumour may exhibit fibro-sarcoma-like configuration.
Low grade central osteosarcoma can be hypo-cellular to moderately cellular and represents with tumour permeation, progression and intramedullary configuration wherein neoplastic cells surround and erode native bone trabeculae and occupy Haversian canal systems. Destruction of bony cortex and infiltration of soft tissue is observed. Scattered mitotic figures may permeate tumour parenchyma. Exceptional, disseminated foci of high-grade neoplasm may appear. Neoplastic cells appear as fibroblast- like, spindle-shaped cells with minimal atypia. Tumour cells are arranged in fascicles or interlacing bundles. Neoplastic bone delineates fibrous dysplasia-like curved, branching or inter-anastomosing bone trabeculae [1,2]. Longitudinal lamellar bone, akin to parosteal osteosarcoma may be observed along with benign, multinucleated giant cells. Focal dissemination of atypical carti-lage may or may not be discerned. Tumour dedifferentiation or metamorphosis into high grade osteosarcoma, high grade undifferentiated pleomorphic sarcoma or fibro-sarcoma is commonly encountered within reoccurring neoplasms, especially within 3 years of surgical resection, although primary tumour may exhibit aforesaid patterns [1,2].
Periosteal osteosarcoma manifests as an ossified tumefaction intensely adherent to native bone cortex after endochondral ossification. Peri-cortical bone configures as dense, mature bone whereas bony spicules radiate from dense, peripheral peri-cortical bone and commingle with hyaline cartilage. Bony spicules demonstrate enlarged, centric vascular cores. Periphery of spicules appear calcified, osseous or chondro-osseous and coalesce with atypical hyaline cartilage. Majority of tumour volume is peripheral. Atypical hyaline cartilage can be classified as grade I to grade III chondrosarcoma. Foci of myxoid alterations can occur [1,2]. Osseous component manifests as an intermediate grade osteosarcoma intermingled with cartilaginous component. Foci of ‘lace-like’ bone may be observed. However, tumefaction is devoid of prominent foci of conventional osteoblastic osteosarcoma. A fibroblastic component comprised of fascicles of spindle-shaped cells with prominent mitosis may be intermixed with aforesaid components [1,2].
High grade surface osteosarcoma simulates conventional, high grade, intramedullary osteosarcoma. Foci of osteoblastic, chondroblastic or fibroblastic tumefaction may be observed. Low grade tumour zones are absent [1,2]. Extra-skeletal osteosarcoma may simulate diverse variants of osteosarcoma. Neoplasm is preponderantly high grade and demonstrates significant cytological atypia with prominent mitotic activity. Intermingled bone may demonstrate trabecular, lace-like, filigree-like or sheet-like configuration [1,2].
TNM staging of osteogenic sarcoma is denominated as [2,3]
Primary tumour Limbs, trunk, skull, facial bones
•TX: Primary tumour cannot be assessed
•T0: No evidence of primary tumour
•T1: Tumour is ≤ 8-centimetre magnitude
•T2: Tumour is > 8-centimetre magnitude
•T3: Primary tumour site exhibits multi-centric neoplasms Spine
•TX: Primary tumour cannot be assessed
•T0: No evidence of primary tumour
•T1: Tumour is confined to single vertebral segment or singular focus of vertebrae or two adjacent vertebral foci
•T2: Tumour is confined to three adjacent vertebral segments
•T3: Tumour is confined to ≥ 4 adjacent vertebral segments or non-adjacent vertebral segments
•T4: Tumour invades spinal canal or great vessels of vertebral column ~T4a: Tumour extends into spinal canal
~T4b: Tumour invades great vessels of vertebral column or impedes vascular outflow Pelvis.
•TX: Primary tumour cannot be assessed
•T0: No evidence of primary tumour or extra-osseous extension
•T1: Tumour manifests singular focus within pelvis
~T1a: Tumour is ≤8-centimetre magnitude.
~T1b: Tumour is > 8-centimetre magnitude.
•T2: Tumour is confined to singular pelvic focus with extra-osseous extension or two pelvic foci with absent extra-osseous extension
~T2a: Tumour is ≤8-centimetre magnitude
~T2b: Tumour is >8-centimetre magnitude.
•T3: Tumour is confined to two pelvic foci with extra-osseous extension
~T3a: Tumour is ≤8-centimetre magnitude.
~T3b: Tumour is > 8-centimetre magnitude.
•T4: Tumour is confined to three pelvic foci or extends beyond sacroiliac joint ~T4a: Tumour invades sacroiliac joint and incriminates sacral neuro-foramina ~T4b: Tumour encases regional vasculature or impedes vascular outflow
Regional lymph nodes
•NX: Regional lymph nodes cannot be assessed
•N0: Regional lymph node metastasis absent
•N1: Regional lymph node metastasis present which is exceptional in a primary bone sarcoma
Distant metastasis
•M0: Distant metastasis absent
•M1: Distant metastasis present
~M1a: Metastasis into pulmonary parenchyma
~M1b: Metastasis into various bones or viscera
Primary bone sarcoma or osteosarcoma is graded as [2,3]
•GX: Tumour grade cannot be identified
•G1: Grade 1 comprised of well differentiated, low grade neoplasms
•G2: Grade 2 composed of moderately differentiated, high grade neoplasms
•G3: Grade 3 constituted of poorly differentiated, high grade neoplasms.
Primary bone sarcoma occurring within spine or pelvis is devoid of pertinent staging. Tumour staging is applicable to neoplasms emerging within skeletal or facial bones, trunk or skull and is designated as [2,3] •stage IA: Tumour is low grade and ≤8-centimetre magnitude(T1). Tumour grade appears as GX or G1. Regional lymph node or distant metastasis is absent(N0,M0). •stage IB: Tumour is low grade and > 8-centimetre magnitude (T2). Tumour grade appears as GX or G1. Primary bone site may depict >one distinct neoplasms. Regional lymph node or distant metastasis is absent(N0,M0). •stage IIA: Tumour is high grade and ≤8-centimetre magnitude(T1). Tumour grade appears as G2 or G3. Regional lymph node or distant metastasis is absent (N0,M0). •stage IIB: Tumour is high grade and > 8-centimetre magnitude (T2).
Tumour grade appears as G2 or G3. Regional lymph node or distant metastasis is absent (N0, M0).
•Stage III: Primary bone site depicts multiple, high-grade tumours( T3). Tumour grade appears as G2 or G3. Regional lymph node or distant metastasis is absent (N0, M0).
•Stage IVA: Tumour magnitude and grade is variable (any T, any G). Regional lymph node metastasis is absent (N0). Distant metastasis into pulmonary parenchyma is discerned(M1a).
•Stage IVB: Tumour magnitude and grade is variable (any T, any G). Regional lymph node or distant metastasis occurs (N1, any M) OR tumour magnitude and grade is variable (any T, any G), regional lymph node metastasis may or may not occur (any N) and tumour metastasis into bone or distant organs besides pulmonary parenchyma (M1b) may ensue [2,3].
Recurrent neoplasm: Tumour reoccurrence may emerge following therapy [2,3]. Osteosarcoma is immune reactive to SATB2, S100 protein, SMA, CD99, osteocalcin. Focal immune reactivity to cytokeratin or EMA is observed. Parosteal and low-grade central osteosarcoma appears immune reactive to MDM2 or CDK4 [3,4]. Osteosarcoma is immune non-reactive to CD31 and CD45. Osteosarcoma requires segregation from diverse neoplasms as chondrosarcoma, dedifferentiated chondrosarcoma, Ewing’s sarcoma, osteochondroma, bizarre parosteal osteochondromatous proliferation or fibrous dysplasia [3,4].
Characteristically, osteosarcoma is suspected upon imaging and disease confirmation is obtained with precise surgical tissue sampling. Upon surgical eradication, excision of core needle or biopsy tract is necessitated in order to circumvent tumour seeding [3,4]. Osteosarcoma demonstrates elevated levels of serum alkaline phosphatase which concurs with disease metastasis and decimated overall survival with declining disease-free survival [3,4]. Upon imaging, an infiltrative, destructive, intraosseous tumefaction appears as an amalgamation of lytic and blastic lesions [3,4]. Permeation of bone cortex and infiltration of circumscribing soft tissue is common. Frequently, centric tumour segment is mineralized. Periosteal reaction emerges as ~sunburst pattern ~Codman’s triangle wherein tumour permeates bone cortex and elevates periosteum followed by bone deposition within the periosteum with configuration of a triangle. Parosteal osteosarcoma defines a tumour mass adherent to bone cortex. Neoplasm depicts a broad base and encases the bone. Tumefaction extends into circumscribing soft tissue. Foci of cortical bone destruction and intramedullary tumour infiltration may occur. Tumour is extensively calcified with prominent centric mineralization [3,4].
Low grade central osteosarcoma demonstrates focal, aggressive features as cortical disruption and tumour extension into circumscribing soft tissue. Tumefaction may appear as benign with well-defined perimeter [3,4]. Periosteal osteosarcoma expounds a ‘soft tissue mass’ centred upon bony cortex wherein neoplasm appears dense around the cortex. Focal cortical thickening with extrinsic scalloping of cortex may be discerned. Periosteal reaction emerges perpendicular to bone cortex, thereby configuring a ‘sunburst pattern’ or ‘Codman’s triangle’. Neoplasm is composed of bony and cartilaginous components along with soft tissue component demonstrating zones of mineralization [3,4]. High grade surface osteosarcoma exhibits tumefaction with broad base situated upon bony surface. The destructive neoplasm enunciates infiltration of bone cortex and soft tissue. Tumour periphery delineates periosteal deposition of bone [3,4]. Appropriate therapy of osteosarcoma is contingent to National Comprehensive Cancer Network (NCCN) guidelines and is exemplified as ~Low grade osteosarcoma or low grade central or parosteal osteosarcoma can be subjected to surgical eradication with removal of wide perimeter of uninvolved tissue. High grade, dedifferentiated neoplasm identifiable upon surgical excision can be treated with adjuvant chemotherapy.
~Periosteal osteosarcoma is subjected to surgical extermination with excision of wide perimeter of normal tissue. Neoadjuvant chemotherapy may be adopted in certain instances. ~High grade intramedullary and surface osteosarcoma is managed with neoadjuvant chemotherapy followed by eradication of wide periphery of uninvolved tissue [3,4]. Adjuvant therapy is preponderantly comprised of chemotherapy. Radiation therapy may be suitably adopted for neoplasms unamenable to surgical resection, contingent to status of tumour periphery [3,4]. Distant metastasis upon initial disease representation can be treated with resection of metastases, tumour resection and subsequent chemotherapy [3,4]. Instances devoid of resection of tumour metastases are appropriately treated with chemotherapy and radiation therapy. ~Extra-skeletal osteosarcoma is managed as a soft tissue sarcoma wherein therapy is contingent to tumour localization, tumour stage and occurrence of neoplasms amenable to surgical resection. ~Post neoadjuvant chemotherapy osteosarcoma requires assessment of therapeutic response denominated as percentage of tumour necrosis or tumour drop out, oedematous scar configured of loose, oedematous to myxoid granulation tissue and focal fi-brosis commingled with mild, chronic inflammatory cell exudate. Remnants of bony matrix with residual tumour cells or nests of tumour cells confined to retraction clefts are commonly discerned [3,4].
Akin to Ewing’s sarcoma, response to chemotherapy is graded as •superior response with > 90% tumour necrosis •grade I: < 50% tumour necrosis •grade II: 50% to 90% tumour necrosis •grade III: 91% to 99% tumour necrosis •grade IV: 100% tumour necrosis. Conventional, high-grade osteosarcoma demonstrates aggressive, localized tumour growth and preliminary haematogenous dissemination with emergence of metastasis into pulmonary parenchyma or bony skeleton [3,4]. Upon induction of contemporary neoadjuvant chemotherapy followed by surgical eradication of tumour, non-metastatic disease exhibits ~70% three-year event free survival. Superior prognostic outcomes are observed with ~ tumour stage T1 or T2 ~low grade tumour (G1) ~tumour confined to accessible sites as upper or lower extremities and amenable to surgical resection. ~localized tumour with absent regional lymph node or distant metastasis ~neoplasms associated with definitive genetic alterations [3,4].
Factors associated with inferior prognostic outcomes are ~tumour metastasis into pulmonary parenchyma, skeletal system, pleura or cardiac muscle ~axial site of tumour emergence, in contrast to tumour occurring within extremities Tumour status following neoadjuvant chemotherapy is associated with significant prognostic outcomes in the absence of metastatic disease wherein ~superior prognosis is observed within grade III or grade IV tumefaction with significantly ameliorated overall and recurrence free survival at 5 years and 10 years. ~inferior prognosis is encountered within grade I and grade II tumefaction with significantly decimated overall and recurrence free survival at 5 years and 10 years [3,4]. Parosteal and low-grade central osteosarcoma exhibit 5-year overall survival of ≥ 90%. Dedifferentiation is encountered in~ 25% instances with inferior prognostic outcomes, akin to conventional high-grade osteosarcoma. Periosteal osteosarcoma exemplifies 5-year overall survival of ~ 89%- and 10-year overall survival at ~ 83% although distant metastasis may ensue [3,4]. High grade surface osteosarcoma delineates prognostic outcomes identical to conventional, high grade, intramedullary osteosarcoma. Extra-skeletal osteosarcoma is associated with 5-year overall survival of ~ 25% [3,4].
References
- Prater S, McKeon B (2022) Osteosarcoma. Stat Pearls International 2022 Treasure Island, Florida, USA.
- Pullan JE, Lotfollahzadeh S (2022) Primary Bone Cancer. Stat Pearls International, Treasure Island, Florida, USA.
- Harris MA, Hawkins CJ (2022) Recent and Ongoing Research into Metastatic Osteosarcoma Treatments. Int J Mol Sci 23(7): 3817.
- Tanaka K, Tsumura H (2019) Eighth edition of the American Joint Committee on Cancer staging system for soft tissue sarcoma of the trunk and extremity: in search of a better staging system. Ann Transl Med 7(Suppl 1):
- Image 1 Courtesy: Wikipedia
- Image 2 Courtesy: Histopathology guru.






























